GI Tables for Anticoagulation Discontinuation & Colonoscopy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laparoscopy As a Diagnostic Tool in Ascites of Unknown Origin: a Retrospective Study Conducted at Kasturba Hospital, Manipal
Research Article Open Access J Surg Volume 8 Issue 3 - March 2018 Copyright © All rights are reserved by Chetan R Kulkarni DOI: 10.19080/OAJS.2018.08.555740 Laparoscopy as a Diagnostic Tool in Ascites of Unknown Origin: A Retrospective Study Conducted at Kasturba Hospital, Manipal Chetan R Kulkarni1*, Badareesh Laxminarayan2 and Annappa Kudva3 1Assistant Professor, Department of Surgery, Kasturba Hospital, Manipal 2Associate Professor, Department of Surgery, Kasturba Hospital, Manipal 3Professor, Department of Surgery, Kasturba Hospital, Manipal Received: February 16, 2018; Published: March 01, 2018 *Corresponding author: Chetan R Kulkarni, Assistant Professor, Department of Surgery, Kasturba Hospital, Manipal, India. Tel: 9535974395;Email: Abstract Background: Laparoscopy as a minimally invasive technique has long played an important role in the evaluation of ascites. Methods: A retrospective analysis was carried out on the record of 80patients who underwent laparoscopy after appropriate investigations had failed to reveal the cause of ascites. Results: Tuberculous peritonitis was reported in 46(57%), malignancies in 18(25%), cirrhosis in 4(5%) and peritonitis of unknown etiology in 8(10%)Conclusion: of patients. Two (2.5%) patients had complications, an Ileal perforation and in other Incisional hernia. Keywords: Ascites;Laparoscopy Diagnostic was Laparoscopy able to diagnose the pathology in 72 (90%) patients with ascites of unknown origin. Introduction Laparoscopy, as a minimally invasive technique has developed rapidly in recent years. Endoscopic examination of conventional laboratory examinations (including ascitic fluid cell count, albumin level, total protein level, Gram stain, culture who termed it as “Celioscopy” [1,2]. The term ‘ascites’ refers to and cytology ) as well as after imaging investigations (including peritoneal cavity was first attempted in 1901 by George Kelling Materialultrasound andand CT Methods scan). -

Practice Parameters for the Treatment of Patients with Dominantly Inherited Colorectal Cancer
Practice Parameters For The Treatment Of Patients With Dominantly Inherited Colorectal Cancer Diseases of the Colon & Rectum 2003;46(8):1001-1012 Prepared by: The Standards Task Force The American Society of Colon and Rectal Surgeons James Church, MD; Clifford Simmang, MD; On Behalf of the Collaborative Group of the Americas on Inherited Colorectal Cancer and the Standards Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons is dedicated to assuring high quality patient care by advancing the science, prevention, and management of disorders and diseases of the colon, rectum, and anus. The standards committee is composed of Society members who are chosen because they have demonstrated expertise in the specialty of colon and rectal surgery. This Committee was created in order to lead international efforts in defining quality care for conditions related to the colon, rectum, and anus. This is accompanied by developing Clinical Practice Guidelines based on the best available evidence. These guidelines are inclusive, and not prescriptive. Their purpose is to provide information on which decisions can be made, rather than dictate a specific form of treatment. These guidelines are intended for the use of all practitioners, health care workers, and patients who desire information about the management of the conditions addressed by the topics covered in these guidelines. Practice Parameters for the Treatment of Patients With Dominantly Inherited Colorectal Cancer Inherited colorectal cancer includes two main syndromes in which predisposition to the disease is based on a germline mutation that may be transmitted from parent to child. -

Endoscopy Rotation Coordination and Goals and Objects Department of Surgery Stanford School of Medicine (8/15/17, Jnl)
Endoscopy Rotation Coordination And Goals and Objects Department of Surgery Stanford School of Medicine (8/15/17, jnl) Rotation Director: James Lau, MD ATTENDINGS and CONTACT INFORMATION Cell Phone E-mail Address James Lau, MD (702) 306-8780 [email protected] Homero Rivas, MD MBA (972) 207-2381 [email protected] Dan Azagury, MD (650) 248-3173 [email protected] Shai Friedland, MD [email protected] Andrew Shelton, MD [email protected] Natalie Kirilcuk, MD [email protected] Cindy Kin, MD [email protected] Laren Becker, MD [email protected] Jennifer Pan, MD [email protected] Suzanne Matsui, MD [email protected] Ramsey Cheung, MD [email protected] KEYPOINT The key for this rotation is that you need to show initiative. TEXT Practical Gastrointestinal Endoscopy: The Fundamentals. Sixth Edition. By Peter B. Cotton, Christopher B. Williams, Robert H. Hawes and Brian P. Saunders. You are responsible for the material to enhance your understanding and supplement your past experiences. Lots of pictures and tips and tricks. Quick read. Copy of text available for purchase on Amazon.com or for check out from the Lane Library. Procedure Schedule Monday Tuesday Wednesday Thursday Friday Laren Becker Jennifer Pan Shelton/Kirilcuk/Kin Ramsey Suzanne (VA (VA Colonoscopy 8:00 am Cheung (VA Matsui (VA Livermore) Livermore) (Stanford Endoscopy) Livermore) Livermore) Every other Tuesday Rivas/Lau alternating Upper/Occasional Lower 1 Endoscopy 9a-1p (Stanford Endoscopy) Suzanne Matsui (VA Livermore) The Staff Drs. Becker, Cheung, Pan, and Matsui are gastroenterologists that perform 75% colonoscopies and 25% upper endoscopies at the Livermore location for the Palo Alto VA. -

Recurrent Pneumothorax Following Abdominal Paracentesis
Postgrad Med J (1990) 66, 319 - 320 © The Fellowship of Postgraduate Medicine, 1990 Postgrad Med J: first published as 10.1136/pgmj.66.774.319 on 1 April 1990. Downloaded from Recurrent pneumothorax following abdominal paracentesis P.J. Stafford Department ofMedicine, Newham General Hospital, Glen Road, Plaistow, London E13, UK. Summary: A 62 year old man presented with abdominal ascites, without pleural effusion, due to peritoneal mesothelioma. He had chronic obstructive airways disease and a past history of right upper lobectomy for tuberculosis. On two occasions abdominal paracentesis was followed within 72 hours by pneumothorax. This previously unreported complication of abdominal paracentesis may be due to increased diaphragmatic excursion following the procedure and should be considered in patients with preexisting lung disease. Introduction Abdominal paracentesis is a widely used palliative right iliac fossa. Approximately 4 litres of turbid therapy for malignant ascites and is generally fluid was drained over 72 hours. The protein accepted as a procedure with few adverse effects: concentration was 46 g/l and microbiology and pneumothorax is not a recognized complication. I cytology were not diagnostic. Laparoscopy re- report a patient with recurrent pneumothorax vealed matted loops of bowel with widespread apparently precipitated by abdominal paracen- peritoneal seedlings. Histological examination of copyright. tesis. these lesions showed malignant mesothelioma of the epithelioid type. The patient suffered a left pneumothorax within Case report 48 hours ofparacentesis (before laparoscopy). This was treated with intercostal underwater drainage A 62 year old civil servant presented with a 6-week but recurred on two attempts to remove the drain, history ofworsening abdominal distension. -

Colon and Rectal Surgery Case Log Instructions Review Committee for Colon and Rectal Surgery
Colon and Rectal Surgery Case Log Instructions Review Committee for Colon and Rectal Surgery Background The ACGME Case Log System is a data depository which provides a mechanism that supports programs in complying with requirements, and also provides a uniform mechanism to verify the clinical education of residents among programs. The Case Log System is designed to capture and categorize a resident’s experience with patient care. It was initially instituted in 2001, and the Review Committee for Colon and Rectal Surgery has required its use by accredited programs since 2005. It is the intention of the Review Committee for Colon and Rectal Surgery that each resident has a reasonably equivalent educational experience to prepare for the practice of the specialty. As part of the process, the case numbers for each resident completing a program are collected and analyzed. To accomplish this complex task, a structured database has been created using standard codes for diagnoses and procedures. The Case Log System helps assess the breadth and depth of clinical experience provided to each colon and rectal surgery resident by his or her program with the ultimate goal of improving the programs themselves. It is the responsibility of the individual residents to accurately and in a timely manner enter their case data. The data entered will be monitored by the program directors and analyzed by the Review Committee. Separate analysis reports are created annually for the Committee, for program directors, and for residents. Additionally, the ACGME provides information regarding individual residents’ experience to the American Board of Colon and Rectal Surgery (ABCRS) as one criterion for their admission to the ABCRS’s exam process. -

FDA Approves ZONTIVITY™ (Vorapaxar), First-In-Class PAR-1
NEWS RELEASE FDA Approves ZONTIVITY™ (vorapaxar), First-in-Class PAR-1 Antagonist, for the Reduction of Thrombotic Cardiovascular Events in Patients with a History of Heart Attack or with Peripheral Arterial Disease 5/12/2014 ZONTIVITY Added to Standard of Care Demonstrated Long-Term Benet Through Three Years Merck (NYSE:MRK), known as MSD outside the United States and Canada, today announced that the U.S. Food and Drug Administration (FDA) has approved ZONTIVITY™ (vorapaxar) for the reduction of thrombotic cardiovascular events in patients with a history of heart attack (myocardial infarction) or in patients with narrowing of leg arteries, called peripheral arterial disease (PAD). For patients with a history of heart attack or with PAD who had no history of stroke or transient ischemic attack (TIA), ZONTIVITY added to standard of care produced a signicant 17 percent relative risk reduction over the three years of the study for the combined events of cardiovascular (CV) death, myocardial infarction (MI), stroke, and urgent coronary revascularization (UCR) [event rate 10.1 percent vs. 11.8 percent for placebo]. For the key secondary composite ecacy endpoint of CV death, MI and stroke alone, ZONTIVITY produced a signicant 20 percent relative risk reduction in these patients [7.9 percent vs. 9.5 percent for placebo]. These results were driven by an 18 percent relative risk reduction in MI [5.4 percent vs. 6.4 percent for placebo] and a 33 percent relative risk reduction in rst stroke [1.2 percent vs. 1.6 percent for placebo]. The prescribing information for ZONTIVITY includes a boxed warning regarding bleeding risk. -
Intestinal and Multiple Organ Transplantation 1679
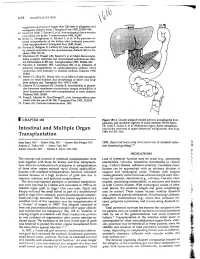
1678 TRANSPLANT ATION euglycemia and survive longer than 200 islets in allogeneic and xenogeneic diabetic hosts. Transplant Proc 1993; 25:953-954. 67. Gotoh M, Maki T, Satomi S, et al: Immunological characteristics of purified islet grafts. Transplantation 1986; 42j387. 68. Klima G, Konigsrainer A, Schmid T, et al: Is the pancreas reo jected independently of the kidney after combined pancreatic renal transplantation? Transplant Proc 1988; 20:665. 69. Prowse 5J, Bellgrau D, Lafferty KJ: Islet allografts are destroyed by disease recurrence in the spontaneously diabetic BB rat. Di abetes 1986; 35:110. 70. Markmann JF, Posselt AM, Bassiri H, et al: Major-histocompat ibility-complex restricted and nonrestricted autoil1'\mune effec tor mechanisms in BB rats. Transplantation 1991; 52:662-667. 71. Navarro X, Kennedy WR, Loewenson RB, et al: Influence of pancreas transplantation on cardiorespiratory reflexes, nerve conduction, and mortality in diabetes mellitus. Diabetes 1990; 39:802. 72. Weber q, Silva FG, Hardy MA, et al: Effect of islet transplan tation on renal function and morphology of short- and long term diabetic rats. Transplant Proc 1979; 11:549. 73. Gotzche 0, Gunderson HJ, Osterby R: Irreversibility of glomer ular basement membrane accumulation despite reversibility of renal hypertrophy with islet transplantation in early diabetes. Diabetes 1981; 30:481. 74. Fung H, Alessini M, Abu-Elmagd K, et al: Adverse effects asso ciated with the use ofFK 506. Transplant Proc 1991; 23:3105. 75. Tzakis AG: Personal communication, 1991. I CHAPTER 185 Figure 185-1. Cluster allograft (shaded portion), including the liver, pancreas, and duodenal segment of small intestine. (From Starzl TE, Todo S. -

Zontivity (Vorapaxar)
HIGHLIGHTS OF PRESCRIBING INFORMATION antiplatelet drugs or with ZONTIVITY as the only antiplatelet These highlights do not include all the information needed to use agent. (2.2) ZONTIVITY safely and effectively. See full prescribing information for ZONTIVITY. --------------------- DOSAGE FORMS AND STRENGTHS --------------------- Tablets: 2.08 mg vorapaxar. (3) ZONTIVITY™ (vorapaxar) Tablets 2.08 mg*, for oral use *Equivalent to 2.5 mg vorapaxar sulfate ------------------------------- CONTRAINDICATIONS ------------------------------- Initial U.S. Approval: 2014 History of stroke, TIA, or ICH. (4.1) Active pathologic bleeding. (4.2) WARNING: BLEEDING RISK See full prescribing information for complete boxed warning. ----------------------- WARNINGS AND PRECAUTIONS ----------------------- • Do not use ZONTIVITY in patients with a history of stroke, Like other antiplatelet agents, ZONTIVITY increases the risk of transient ischemic attack (TIA), or intracranial hemorrhage (ICH); bleeding. (5.1) or active pathological bleeding. (4.1, 4.2) Avoid use with strong CYP3A inhibitors or inducers. (5.2) • Antiplatelet agents, including ZONTIVITY, increase the risk of bleeding, including ICH and fatal bleeding. (5.1) ------------------------------ ADVERSE REACTIONS ------------------------------ Bleeding, including life-threatening and fatal bleeding, is the most ----------------------------INDICATIONS AND USAGE ---------------------------- commonly reported adverse reaction. (6.1) ZONTIVITY is a protease-activated receptor-1 (PAR-1) antagonist indicated for the reduction of thrombotic cardiovascular events in To report SUSPECTED ADVERSE REACTIONS, contact Merck patients with a history of myocardial infarction (MI) or with peripheral Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877 arterial disease (PAD). ZONTIVITY has been shown to reduce the rate 888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. of a combined endpoint of cardiovascular death, MI, stroke, and urgent coronary revascularization. -

Guidelines on the Management of Ascites in Cirrhosis
Downloaded from gut.bmjjournals.com on 25 September 2006 Guidelines on the management of ascites in cirrhosis K P Moore and G P Aithal Gut 2006;55;1-12 doi:10.1136/gut.2006.099580 Updated information and services can be found at: http://gut.bmjjournals.com/cgi/content/full/55/suppl_6/vi1 These include: References This article cites 148 articles, 21 of which can be accessed free at: http://gut.bmjjournals.com/cgi/content/full/55/suppl_6/vi1#BIBL Email alerting Receive free email alerts when new articles cite this article - sign up in the box at the service top right corner of the article Topic collections Articles on similar topics can be found in the following collections Liver, including hepatitis (945 articles) Notes To order reprints of this article go to: http://www.bmjjournals.com/cgi/reprintform To subscribe to Gut go to: http://www.bmjjournals.com/subscriptions/ Downloaded from gut.bmjjournals.com on 25 September 2006 vi1 GUIDELINES Guidelines on the management of ascites in cirrhosis K P Moore, G P Aithal ............................................................................................................................... Gut 2006;55(Suppl VI):vi1–vi12. doi: 10.1136/gut.2006.099580 1.0 INTRODUCTION N Grade 1 (mild). Ascites is only detectable by ultrasound examination. Ascites is a major complication of cirrhosis,1 occurring in 50% of patients over 10 years of N Grade 2 (moderate). Ascites causing moderate follow up.2 The development of ascites is an symmetrical distension of the abdomen. important landmark in the natural history of N Grade 3 (large). Ascites causing marked cirrhosis as it is associated with a 50% mortality abdominal distension. -

Procedure Coding in ICD-9-CM and ICD- 10-PCS
Procedure Coding in ICD-9-CM and ICD- 10-PCS ICD-9-CM Volume 3 Procedures are classified in volume 3 of ICD-9-CM, and this section includes both an Alphabetic Index and a Tabular List. This volume follows the same format, organization and conventions as the classification of diseases in volumes 1 and 2. ICD-10-PCS ICD-10-PCS will replace volume 3 of ICD-9-CM. Unlike ICD-10-CM for diagnoses, which is similar in structure and format as the ICD-9-CM volumes 1 and 2, ICD-10-PCS is a completely different system. ICD-10-PCS has a multiaxial seven-character alphanumeric code structure providing unique codes for procedures. The table below gives a brief side-by-side comparison of ICD-9-CM and ICD-10-PCS. ICD-9-CM Volume3 ICD-10-PCS Follows ICD structure (designed for diagnosis Designed and developed to meet healthcare coding) needs for a procedure code system Codes available as a fixed or finite set in list form Codes constructed from flexible code components (values) using tables Codes are numeric Codes are alphanumeric Codes are 3-4 digits long All codes are seven characters long ICD-9-CM and ICD-10-PCS are used to code only hospital inpatient procedures. Hospital outpatient departments, other ambulatory facilities, and physician practices are required to use CPT and HCPCS to report procedures. ICD-9-CM Conventions in Volume 3 Code Also In volume 3, the phrase “code also” is a reminder to code additional procedures only when they have actually been performed. -

AASLD Position Paper : Liver Biopsy
AASLD POSITION PAPER Liver Biopsy Don C. Rockey,1 Stephen H. Caldwell,2 Zachary D. Goodman,3 Rendon C. Nelson,4 and Alastair D. Smith5 This position paper has been approved by the AASLD and College of Cardiology and the American Heart Associa- represents the position of the association. tion Practice Guidelines3).4 Introduction Preamble Histological assessment of the liver, and thus, liver bi- These recommendations provide a data-supported ap- opsy, is a cornerstone in the evaluation and management proach. They are based on the following: (1) formal re- of patients with liver disease and has long been considered view and analysis of the recently published world to be an integral component of the clinician’s diagnostic literature on the topic; (2) American College of Physi- armamentarium. Although sensitive and relatively accu- cians Manual for Assessing Health Practices and De- rate blood tests used to detect and diagnose liver disease signing Practice Guidelines1; (3) guideline policies, have now become widely available, it is likely that liver including the AASLD Policy on the Development and biopsy will remain a valuable diagnostic tool. Although Use of Practice Guidelines and the American Gastro- histological evaluation of the liver has become important enterological Association Policy Statement on Guide- in assessing prognosis and in tailoring treatment, nonin- lines2; and (4) the experience of the authors in the vasive techniques (i.e., imaging, blood tests) may replace specified topic. use of liver histology in this setting, particularly with re- Intended for use by physicians, these recommenda- gard to assessment of the severity of liver fibrosis.5,6 Sev- tions suggest preferred approaches to the diagnostic, ther- eral techniques may be used to obtain liver tissue; a table apeutic, and preventive aspects of care. -

Chyle Leak Post Laparoscopic Cholecystectomy: a Case Report, Literature Review and Management Options
7 Case Report Page 1 of 7 Chyle leak post laparoscopic cholecystectomy: a case report, literature review and management options Ferdinand Ong1, Amitabha Das1, Kheman Rajkomar2 1Department of Upper Gastrointestinal Surgery, Liverpool Hospital, Sydney, NSW, Australia; 2Department of Upper Gastrointestinal Surgery, Bankstown-Lidcombe Hospital, Sydney, NSW, Australia Correspondence to: Dr. Kheman Rajkomar. Eldridge Road, Bankstown-Lidcombe Hospital, Bankstown NSW 2200, Sydney, Australia. Email: [email protected]. Abstract: Chyle leak after a laparoscopic cholecystectomy (LC) is very rarely reported. However, it is needs to be recognised promptly and managed as otherwise it can lead to further metabolic and infective complications. We present the case of a 48 years old man who was admitted with ultrasound proven acute calculous cholecystitis. His vital signs were within normal range but his murphy’s sign was positive. His white cell count (WCC) and liver function tests were within normal limit. He underwent an uneventful standard LC with cholangiography during the same admission with no anomalous biliary or hepatic arterial anatomy noted during the procedure. Post operatively he was noted to have 125 mL of white fluid in his drain. The fluid triglyceride was 23.2 mmol/L, cholesterol level was 2.8 mmol/L, and drain/serum triglyceride of 15.5, hence confirming it to be chyle. He was clinically otherwise very well. He was managed conservatively as a low volume chyle leak with a fat free diet. The triglyceride content in the drain effluent decreased to 1.3mmol/L by day 6 and the fluid turned straw coloured in that interval. The drain was removed and the patient discharged home without any further issues.