Master of Forestry Science Carbon Sequestered by Native Restoration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Novel Habitats, Rare Plants and Root Traits
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. Novel Habitats, Rare Plants and Roots Traits A thesis submitted in partial fulfilment of the requirements for the Degree of Master of Applied Science at Lincoln University by Paula Ann Greer Lincoln University 2017 Abstract of a thesis submitted in partial fulfilment of the requirements for the Degree of Master of Applied Science. Abstract Novel habitats, rare plants and root traits. by Paula Ann Greer The loss of native plant species through habitat loss has been happening in NZ since the arrival of humans. This is especially true in Canterbury where less than 1% of the lowland plains are believed to be covered in remnant native vegetation. Rural land uses are changing and farm intensification is creating novel habitats, including farm irrigation earth dams. Dam engineers prefer not to have plants growing on dams. Earth dams are consented for 100 years, they could be used to support threatened native plants. Within the farm conversion of the present study dams have created an average of 1.7 hectares of ‘new land’ on their outside slope alone, which is the area of my research. -

Here Before We Humans Were and Their Relatives Will Probably Be Here When We Are Gone
The ‘mighty tōtara’ is one of our most extraordinary trees. Among the biggest and oldest trees in the New Zealand forest, the heart of Māori carving and culture, trailing no. 8 wire as fence posts on settler farms, clambered up in the Pureora protests of the 1980s: the story of New Zealand can be told through tōtara. Simpson tells that story like nobody else could. In words and pictures, through waka and leaves, farmers and carvers, he takes us deep inside the trees: their botany and evolution, their role in Māori life and lore, their uses by Pākehā, and their current status in our environment and culture. By doing so, Simpson illuminates the natural world and the story of Māori and Pākehā in this country. Our largest trees, the kauri Tāne Mahuta and the tōtara Pouakani, are both thought to be around 1000 years old. They were here before we humans were and their relatives will probably be here when we are gone. Tōtara has been central to life in this country for thousands of years. This book tells a great tree’s story, and that is our story too. Philip Simpson is a botanist and author of Dancing Leaves: The Story of New Zealand’s Cabbage Tree, Tī Kōuka (Canterbury University Press, 2000) and Pōhutukawa and Rātā: New Zealand’s Iron-hearted Trees (Te Papa Press, 2005). Both books won Montana Book Awards in the Environment category and Pōhutukawa and Rātā also won the Montana Medal for best non-fiction book. Simpson is unique in his ability to combine the scientific expertise of the trained botanist with a writer’s ability to understand the history of Māori and Pākehā interactions with the environment. -

Environmental Pest Plants
RESTORATION PLAN FOR THE SPENCER ROAD PART OF THE LAKE TARAWERA CATCHMENT R4152c RESTORATION PLAN FOR THE SPENCER ROAD PART OF THE LAKE TARAWERA CATCHMENT Contract Report No. 4152c February 2017 Updated February 2019 Project Team: Richard Gillies - Fieldwork, report author Jennifer Murray - Fieldwork, report author Sarah Beadel - Field assessment, project management, report author, peer review Prepared for: Lake Tarawera Ratepayers Association and Tarawera Landcare 2115 99 SALA STREET, WHAKAREWAREWA, 3010, P.O. BOX 7137, TE NGAE, ROTORUA 3042 Ph 07-343-9017; Fax 07-343-9018, email [email protected], www.wildlands.co.nz EXECUTIVE SUMMARY Tarawera Landcare 2115, via the Lake Tarawera Ratepayers Association, has signed a MoU with Rotorua Lakes Council regarding the management of Council land along the margins of Lake Tarawera between Otumutu Lagoon and Te Toroa Point. The site is c.6.5 km long and covers 24 ha. The Lake Tarawera Ratepayers Association has commissioned this plan to guide ecological management there. A separate plan has been developed for Māori land on Kariri Point. A review of background information and ecological surveys and assessments of the study area were undertaken during October-December 2016. Indigenous forest dominated by māhoe, mamaku, kāmahi, fivefinger and, in places, pōhutukawa and kānuka, occupies around 40% of the study area, and is representative of the vegetation regenerating after the 1886 Tarawera eruption. A distinctive feature is the presence of healthy numbers of the mistletoe Tupeia antarctica, an At Risk species, from Cliff Road Reserve northwards, attributable to ongoing possum control by the Tarawera community. Lakeshore vegetation provides roosting and nesting habitat for waterbirds, particularly dabchick, a Threatened endemic waterbird present along the entire shoreline. -

Sanctuary Mountain Maungatautari Restoration Plan 2019–2029
Sanctuary Mountain Maungatautari Restoration Plan 2019–2029 Prepared for: Maungatautari Ecological Island Trust June 2019 Sanctuary Mountain Maungatautari Restoration Plan 2019–2029 Contract Report: LC3464 John Innes, Corinne Watts Manaaki Whenua – Landcare Research Bruce Burns University of Auckland With contributions from other members of, and advisors to, the Maungatautari Scientific and Technical Advisory Panel: Aaron Barnsdall (Mana Whenua Trustee) Geoff Churchill (Maungatautari Ecological Island Trust) Professor Bruce Clarkson (University of Waikato) Poto Davies (Maungatautari Mana Whenua) Dr Catherine Kirby (University of Waikato) Cheridan Mathers (Hamilton Zoo) Dr Shelley Langton-Myers (EcoQuest Education Foundation) Robyn Nightingale (Maungatautari Mana Whenua) Dr Kate Richardson (Waikato Regional Council) Tony Roxburgh (Waipa District Council) Alan Saunders (Waikato Regional Council) Dr Andrew Styche (Department of Conservation) Dr Kiri Wallace (University of Waikato) Dr Janelle Ward (Maungatautari Ecological Island Trust) Reviewed by: Approved for release by: Robyn Simcock Gary Houliston Scientist Portfolio Leader – Enhancing Biodiversity Manaaki Whenua – Landcare Research Manaaki Whenua – Landcare Research Disclaimer This report has been prepared by Manaaki Whenua – Landcare Research for Maungatautari Ecological Island Trust. If used by other parties, no warranty or representation is given as to its accuracy and no liability is accepted for loss or damage arising directly or indirectly from reliance on the information in it. -

Forest Ecosystems of the Wellington Region December 2018
Forest Ecosystems of the Wellington Region December 2018 Forest ecosystems of the Wellington Region December 2018 Nick Singers, Philippa Crisp and Owen Spearpoint For more information, contact the Greater Wellington Regional Council: Wellington Masterton GW/ESCI-G-18-164 PO Box 11646 PO Box 41 December 2018 T 04 384 5708 T 06 378 2484 F 04 385 6960 F 06 378 2146 www.gw.govt.nz www.gw.govt.nz www.gw.govt.nz [email protected] DISCLAIMER This report has been prepared by Environmental Science staff of Greater Wellington Regional Council (GWRC) and as such does not constitute Council policy. In preparing this report, the authors have used the best currently available data and have exercised all reasonable skill and care in presenting and interpreting these data. Nevertheless, GWRC does not accept any liability, whether direct, indirect, or consequential, arising out of the provision of the data and associated information within this report. Furthermore, as GWRC endeavours to continuously improve data quality, amendments to data included in, or used in the preparation of, this report may occur without notice at any time. GWRC requests that if excerpts or inferences are drawn from this report for further use, due care should be taken to ensure the appropriate context is preserved and is accurately reflected and referenced in subsequent written or verbal communications. Any use of the data and information enclosed in this report, for example, by inclusion in a subsequent report or media release, should be accompanied by an acknowledgement of the source. The report may be cited as: Singers N., Crisp P. -

Full Production Catalogue 2021
FULL PRODUCTION CATALOGUE 2021 POT SIZE PLANT NAME COMMON NAME DESCRIPTION (Litre) Acaena inermis 'Purpurea' Purple Bidibidi Spreading mat plant with purple leaves, white flowers. RX90 Acaena novae zelandiae Piripiri Tight green groundcover with white flowers & large bright red seed heads. RX90 Agathis australis Kauri Iconic long lived New Zealand tree 4 Agathis australis Kauri Iconic long lived New Zealand tree 9 Alectryon excelsus Titoki Handsome green foliage, black seed held in brilliant red fruits, fine specimen tree. Light frosts. PP/HRT Alectryon excelsus Titoki Handsome green foliage, black seed held in brilliant red fruits, fine specimen tree. Light frosts. 4 Alseuosmia macrophylla Toropapa Uncommon shrub of shady forests with strongly scented red/white flowers & large red fruit. 2.5 Anemanthele lessoniana Gossamer Grass Vigorous grass, flowing golden green and reddish-brown leaves, fine feathery pink flowerhead. RT Apodasmia similis Oi oi, Jointed rush Bluish green evergreen rush, esp. suitable for planting by water. PP/HRT Aristotelia serrata Wineberry Quick growing tree with large shining leaves & rose pink flowers and fruits RT/PP Arthropodium bifurcatum Renga renga lily Bold foliage plant with broad leaves & white flower spikes. Light frosts only, or understory. RT/HRT Arthropodium bifurcatum Renga renga lily Bold foliage plant with broad leaves & white flower spikes. Light frosts only, or understory. 2.5 Arthropodium candidum Miniature renagrenga Deciduous tiny renga renga with green foliage and delicate white flowers. RX90 Arthropodium cirratum Renga renga lily Bold foliage plant with finer blades & white flower spikes. Light frosts only, or understory. HRT Asplenium bulbiferum Hen & Chicken fern Graceful deep green fronds that develop tiny bulbils or “Chickens”. -

Quarterly Changes
Plant Names Database: Quarterly changes 31 August 2015 © Landcare Research New Zealand Limited 2015 This copyright work is licensed under the Creative Commons Attribution 3.0 New Zealand license. Attribution if redistributing to the public without adaptation: "Source: Landcare Research" Attribution if making an adaptation or derivative work: "Sourced from Landcare Research" http://dx.doi.org/doi:10.7931/P1301R CATALOGUING IN PUBLICATION Plant names database: quarterly changes [electronic resource]. – [Lincoln, Canterbury, New Zealand] : Landcare Research Manaaki Whenua, 2014- . Online resource Quarterly November 2014- ISSN 2382-2341 I.Manaaki Whenua-Landcare Research New Zealand Ltd. II. Allan Herbarium. Citation and Authorship Wilton, A.D.; Schönberger, I.; Gibb, E.S.; Boardman, K.F.; Breitwieser, I.; Cochrane, M.; Dawson, M.I.; de Pauw, B.; Fife, A.J.; Ford, K.A.; Glenny, D.S.; Heenan, P.B.; Korver, M.A.; Novis, P.M.; Redmond, D.N.; Smissen, R.D. Tawiri, K. (2015) Plant Names Database: Quarterly changes. August 2015. Lincoln, Manaaki Whenua Press. This report is generated using an automated system and is therefore authored by the staff at the Allan Herbarium who currently contribute directly to the development and maintenance of the Plant Names Database. Authors are listed alphabetically after the third author. Authors have contributed as follows: Leadership: Wilton, Heenan, Breitwieser Database editors: Wilton, Schönberger, Gibb Taxonomic and nomenclature research and review: Schönberger, Gibb, Wilton, Breitwieser, Dawson, Ford, Fife, Glenny, Heenan, Novis, Redmond, Smissen Information System development: Wilton, De Pauw, Cochrane Technical support: Boardman, Korver, Redmond, Tawiri Disclaimer The Plant Names Database is being updated every working day. We welcome suggestions for improvements, concerns, or any data errors you may find. -
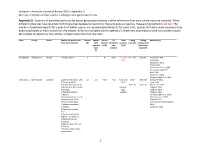
Jacobsen—American Journal of Botany 2021—Appendix S1 Diversity in Conduit and Pit Structure Among Extant Gymnosperm Taxa
Jacobsen—American Journal of Botany 2021—Appendix S1 Diversity in conduit and pit structure among extant gymnosperm taxa Appendix S1. Summary of pit membrane traits for extant gymnosperm genera and the references from which these traits are compiled. When different references have reported conflicting presence/absence reports for the same genus or species, these are highlighted in red text. The number of published reports for a given trait within a genus are included parenthetically for some traits. Species that were newly examined from dried wood blocks or fresh samples for the present review are indicated with an asterisk (*); these new examinations were not counted toward the number of reports nor the numbers of observations for trait characters. Phyla Family Genus Species for which traits Reports Species Vessels Pit Torus Pitting Pitting Reference(s) have been reported (#) with present membrane presence (-seriate) arrangement reported (Y/N) type (Y/N) (alternate/ traits (A/B/C) opposite) (#) Ginkgophyta Ginkgoaceae Ginkgo *Ginkgo biloba L. 9 1 N B (2) Y (1) and uni- & bi- opposite Penhallow 1907, N (6) Liese 1965, Bauch et al. 1972, Timell 1978, Schulte and Gibson 1988, Yin and Xiao-Mei 1992, Dute 1994, Hacke et al. 2004, Schweingruber et al. 2006 Gnetophyta Ephedraceae Ephedra Ephedra americana Humb. 22 12 Y (17) A (2) Y (17) and multi- alternate MacDuffie 1921, & Bonpl. ex Willd., N (2) Liese 1965, Ephedra distachya subsp. (vessels) (vessels) Bauch et al. 1972, helvetica (C.A.Mey.) Asch. (conduit- Carlquist 1990, & Graebn., type Carlquist 1992, Ephedra fasciculata specific) Carlquist 1996, A.Nelson, Schweingruber et al. 2006, Ephedra foeminea Forssk., Motomura et al. -

Podocarpus Laetus
Podocarpus laetus COMMON NAME Mountain totara, Hall’s totara, thin-barked totara, totara-kiri-kotukutuku SYNONYMS Podocarpus hallii Kirk; Podocarpus cunninghamii Colenso FAMILY Podocarpaceae AUTHORITY Podocarpus laetus Hooibr. ex Endl. FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes ENDEMIC GENUS No ENDEMIC FAMILY No STRUCTURAL CLASS Podocarpus cunninghamii. Photographer: Trees & Shrubs - Gymnosperms Wayne Bennett NVS CODE PODCUN CHROMOSOME NUMBER 2n = 34 CURRENT CONSERVATION STATUS 2012 | Not Threatened PREVIOUS CONSERVATION STATUSES 2009 | Not Threatened 2004 | Not Threatened DISTRIBUTION Endemic. North, South and Stewart Islands. HABITAT Lowland, montane to lower subalpine forest (but notably more common in montane forest). Often found on impoverished soils, immature (skeletal) soils, or sites that are naturally stressed by drought or extreme temperature fluctuations. Photo of Halls totara bark. Photographer: DoC FEATURES Robust dioecious conifer up to 20 m tall. Trunk stout, 1-1.5 m diam., clad in papery, thin, freely flaking reddish-grey bark. Trunk without branches at base, branches slender, erect, spreading or somewhat drooping. Leaf bud significantly broader than the diam., of the branchlet, surrounded by caducous, papery, ovate bracts. Leaves yellow-green, green, or brownish-green, erect, leathery; juvenile 25-50 x 4-5 mm, adults 20-30 x 3-4 mm., narrow- linear to linear-lanceolate, acute to acuminate, apex very pungent, mid-vein distinct. Male cones (strobili) axillary, 10-25 mm, solitary or up to 5 on a common peduncle. Female branchlets axillary, ovules solitary or paired. Receptacle of 2-4 scales, irregularly elliptic-oblong to obovate-oblong, maturing as a red, swollen, succulent, sweet tasting “fruit” this surmounted by a 1(-2) elliptic, elliptic-oblong or ovate-oblong, (5-)6.5-8.5 mm long, grey nut brown or dark brown (green when fresh) seed. -

Flora of New Zealand Mosses
FLORA OF NEW ZEALAND MOSSES ORTHOTRICHACEAE A.J. FIFE Fascicle 31 – FEBRUARY 2017 © Landcare Research New Zealand Limited 2017. Unless indicated otherwise for specific items, this copyright work is licensed under the Creative Commons Attribution 4.0 International licence Attribution if redistributing to the public without adaptation: “Source: Landcare Research” Attribution if making an adaptation or derivative work: “Sourced from Landcare Research” See Image Information for copyright and licence details for images. CATALOGUING IN PUBLICATION Fife, Allan J. (Allan James), 1951- Flora of New Zealand [electronic resource] : mosses. Fascicle 31, Orthotrichaceae / Allan J. Fife. -- Lincoln, N.Z. : Manaaki Whenua Press, 2017. 1 online resource ISBN 978-0-947525-04-0 (pdf) ISBN 978-0-478-34747-0 (set) 1.Mosses -- New Zealand -- Identification. I. Title. II. Manaaki Whenua-Landcare Research New Zealand Ltd. UDC 582.344.924 (931) DC 588.20993 DOI: 10.7931/B1J01C This work should be cited as: Fife, A.J. 2017: Orthotrichaceae. In: Breitwieser, I.; Wilton, A.D. Flora of New Zealand - Mosses. Fascicle 31. Manaaki Whenua Press, Lincoln. http://dx.doi.org/10.7931/B1J01C Cover image: Orthotrichum assimile, habit with capsules, moist. Redrawn by Rebecca Wagstaff with permission from Lewinsky (1984). Contents Introduction..............................................................................................................................................1 Typification...............................................................................................................................................1 -

Indigenous Terrestrial and Wetland Ecosystems of Auckland
Indigenous terrestrial and wetland ecosystems of Auckland Recommended citation: Singers, N.; Osborne, B.; Lovegrove, T.; Jamieson, A.; Boow, J.; Sawyer, J.; Hill, K.; Andrews, J.; Hill, S.; Webb, C. 2017. Indigenous terrestrial and wetland ecosystems of Auckland. Auckland Council. Cover image: Forest understorey on the Kohukohunui Track, Hunua Ranges. Jason Hosking. ISBN 978-0-9941351-6-2 (Print) ISBN 978-0-9941351-7-9 (PDF) © Auckland Council, 2017. All photographs are copyright of the respective photographer. Indigenous terrestrial and wetland ecosystems of Auckland Singers, N.; Osborne, B.; Lovegrove, T.; Jamieson, A.; Boow, J.; Sawyer, J.; Hill, K.; Andrews, J.; Hill, S.; Webb, C. Edited by Jane Connor. 2017. 3 This guide is dedicated to John Sawyer whose vision initiated the project and who made a substantial contribution to the publication prior to leaving New Zealand. Sadly, John passed away in 2015. John was passionate about biodiversity conservation and brought huge energy and determination to the projects he was involved with. He was generous with his time, knowledge and support for his many friends and colleagues. INDIGENOUS TERRESTRIAL AND WETLAND ECOSYSTEMS OF AUCKLAND Contents Indigenous terrestrial and wetland ecosystems of Auckland Introduction 7 Origins of a national ecosystem classification system 10 Mapping Auckland’s ecosystems 10 Threatened ecosystems 11 Threatened ecosystem assessments in Auckland 11 Introduction to the ecosystem descriptions 13 Forest ecosystems 15 WF4: Pōhutukawa, pūriri, broadleaved forest [Coastal -

Investigating the Habitat Suitability of Maungatautari Ecological Island for the Reintroduction of Kākāpō (Strigops Habroptilus)
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. Investigating the habitat suitability of Maungatautari Ecological Island for the reintroduction of kākāpō (Strigops habroptilus) A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science in Conservation Biology Massey University, Palmerston North New Zealand Alexandra J. Hurley 2017 “They are our national monuments. They are our Tower of London, our Arc de Triomphe, our pyramids. We don’t have this ancient architecture that we can be proud of and swoon over in wonder, but what we do have is something that is far, far older than that. No one else has kiwi, no one else has kākāpō. They have been around for millions of years, if not thousands of millions of years. And once they are gone, they are gone forever. And it’s up to us to make sure they never die out." Don Merton Abstract The kākāpō (Strigops habroptilus) is a large, flightless parrot endemic to New Zealand which was once abundant across mainland New Zealand. However, this nocturnal bird species is now listed as critically endangered with a population of approximately 154 individuals. Kākāpō are currently only found on four offshore, predator-free islands where kākāpō were not found historically - Whenua Hou/Codfish Island, Hauturu-o-Toi/Little Barrier Island, Anchor Island and an unnamed island in Fiordland.