Vitamin K and Osteoporosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Downregulation of the Clotting Cascade by the Protein C
Downregulation of the clotting cascade by the protein C F. Stavenuiter, E.A.M. Bouwens, L.O. Mosnier I Simposio Conjunto EHA - SAH Department of Molecular and Experimental Medicine, The Scripps Research Institute, La Jolla, CA, USA HEMATOLOGÍA, Vol.17 Número Extraordinario XXI CONGRESO E-mail: [email protected] Octubre 2013 Abstract APC mutants, which provide unique insights into The protein C pathway provides important biologi- the relative contributions of APC’s anticoagulant or cal activities to maintain the fluidity of the circula- cytoprotective activities to the beneficial effects of tion, prevent thrombosis, and protect the integrity APC in various murine injury and disease models. of the vasculature in response to injury. Activated Because of its multiple physiological and pharmaco- protein C (APC), in concert with its co-factors and logical activities, the anticoagulant and cytoprotec- cell receptors, assembles in specific macromolecular tive protein C pathway have important implications complexes to provide efficient proteolysis of multiple for the (patho)physiology of vascular disease and substrates that result in anticoagulant and cytopro- for translational research exploring novel therapeu- tective activities. Numerous studies on APC’s struc- tic strategies to combat complex medical disorders ture-function relation with its co-factors, cell recep- such as thrombosis, inflammation, ischemic stroke tors, and substrates provide valuable insights into the and neurodegenerative disease. molecular mechanisms and presumed assembly of Learning goals the macromolecular complexes that are responsible At the conclusion of this activity, participants should for APC’s activities. These insights allow for molecu- know that: lar engineering approaches specifically targeting the - the protein C pathway provides multiple im- interaction of APC with one of its substrates or co- portant functions to maintain a regulated bal- factors. -

Collagen and Elastin Fibres
J Clin Pathol: first published as 10.1136/jcp.s3-12.1.49 on 1 January 1978. Downloaded from J. clin. Path., 31, Suppl. (Roy. Coll. Path.), 12, 49-58 Collagen and elastin fibres A. J. BAILEY From the Agricultural Research Council, Meat Research Institute, Langford, Bristol Although an understanding of the intracellular native collagen was generated from type I pro- biosynthesis of both collagen and elastin is of collagen. Whether this means that the two pro- considerable importance it is the subsequent extra- collagens are converted by different enzyme systems cellular changes involving fibrogenesis and cross- and the type III enzyme was deficient in these linking that ensure that these proteins ultimately fibroblast cultures, or that the processing of pro become the major supporting tissues of the body. type III is extremely slow, is not known. The latter This paper summarises the formation and stability proposal is consistent with the higher proportion of collagen and elastin fibres. of soluble pro type III extractable from tissue (Lenaers and Lapiere, 1975; Timpl et al., 1975). Collagen Basement membrane collagens, on the other hand, do not form fibres and this property may be The non-helical regions at the ends of the triple due to the retention of the non-helical extension helix of procollagen probably provide a number of peptides (Kefalides, 1973). In-vivo biosynthetic different intracellular functions-that is, initiating studies showing the absence of any extension peptide rapid formation of the triple helix; inhibiting intra- removal support this (Minor et al., 1976), but other cellular fibrillogenesis; and facilitating transmem- workers have reported that there is some cleavage brane movement. -

Suppression of Prostate Tumor Cell Growth by Stromal Cell Prostaglandin D Synthase–Derived Products
Research Article Suppression of Prostate Tumor Cell Growth by Stromal Cell Prostaglandin D Synthase–Derived Products Jeri Kim,1 Peiying Yang,2 Milind Suraokar,3 Anita L. Sabichi,3 Norma D. Llansa,3 Gabriela Mendoza,3 Vemparalla Subbarayan,3 Christopher J. Logothetis,1 Robert A. Newman,2 Scott M. Lippman,3 and David G. Menter3 Departments of 1Genitourinary Medical Oncology, 2Experimental Therapeutics, and 3Clinical Cancer Prevention, The University of Texas M.D. Anderson Cancer Center, Houston, Texas Abstract seminal fluid (10). Once PGD2 is made, it forms derivative Stromal-epithelial interactions and the bioactive molecules compounds, most of which can transactivate the peroxisome g g produced by these interactions maintain tissue homeostasis proliferator–activated receptor (PPAR ). One PGD2 derivative, 15-deoxy-D12,14-prostaglandin J (15-d-PGJ ), can slow the growth and influence carcinogenesis. Bioactive prostaglandins pro- 2 2 duced by prostaglandin synthases and secreted by the prostate and induce the partial differentiation of selected cancer cells (12). D12,14 into seminal plasma are thought to support reproduction, but Another PGD2 derivative, 15-deoxy- -PGD2 (15-d-PGD2), has g their endogenous effects on cancer formation remain unre- also been shown to stimulate PPAR transactivation in RAW 264.7 solved. No studies to date have examined prostaglandin cell macrophage cultures as effectively as 15-d-PGJ2 (13). L-PGDS enzyme production or prostaglandin metabolism in normal also binds tritiated testosterone and may play a role in androgen prostate stromal cells. Our results show that lipocalin-type transport (14). In castrated rats, testosterone proprionate induces prostaglandin D synthase (L-PGDS) and prostaglandin D L-PGDS synthesis in the epididymis (15). -

Outpatient Acne Care Guideline
Outpatient Acne Care Guideline Severity Mild Moderate Severe < 20 comedones or < 20-100 comedones or 15-50 > 5 cysts, >100 comedones, or inflammatory lesions inflammatory lesions >50 inflammatory lesions Initial Treatment Initial Treatment Initial Treatment Benzoyl Peroxide (BP) or Topical Combination Therapy Combination Therapy Topical Retinoid Retinoid + BP Oral antibiotic or OR + (Retinoid + Antibiotic) + BP Topical retinoid Topical Combination Therapy or + BP + Antibiotic Retinoid + (BP + Antibiotic) or OR BP Retinoid + BP Oral antibiotic + topical retinoid + +/- or BP Topical antibiotic Retinoid + Antibiotic + BP or Topical Dapsone IF Inadequate Response IF Inadequate Response IF Inadequate Consider dermatology Response referral Change topical retinoid Consider changing oral concentrations, type and/or antibiotic formulation AND or Add BP or retinoid, if not already Change topiocal combination Consider isotretinoin prescribed therapy Consider hormone therapy or and/or (females) Change topical retinoid Add or change oral antibiotic concentrations, type and/or or formulation Consider isotretinoin Additional Considerations or Consider hormone therapy (females) Change topical comination Previous treatment/history Side effects therapy Costs Psychosocial impact Vehicle selection Active scarring Ease of use Regimen complexity Approved Evidence Based Medicine Committee 1-18-17 Reassess the appropriateness of Care Guidelines as condition changes. This guideline is a tool to aid clinical decision making. It is not a standard of care. The physician should deviate from the guideline when clinical judgment so indicates. GOAL: Pediatricians should initiate treatment for cases of “Mild” to “Severe” acne (see algorithms attached). Pediatricians should also counsel patients in order to maximize adherence to acne treatment regimens: 1. Realistic expectations. Patients should be counseled that topical therapies typically take up to 6-8 weeks to start seeing results. -

Evaluation of Elastin/Collagen Content in Human Dermis In-Vivo by Multiphoton Tomography—Variation with Depth and Correlation with Aging
Cosmetics 2014, 1, 211-221; doi:10.3390/cosmetics1030211 OPEN ACCESS cosmetics ISSN 2079-9284 www.mdpi.com/journal/cosmetics Article Evaluation of Elastin/Collagen Content in Human Dermis in-Vivo by Multiphoton Tomography—Variation with Depth and Correlation with Aging Jean-Christophe Pittet 1,*, Olga Freis 2,†, Marie-Danielle Vazquez-Duchêne 2,†, Gilles Périé 2,† and Gilles Pauly 2,† 1 Orion Concept, 100 Rue de Suède, 37100 Tours, France 2 BASF Beauty Care Solutions France SAS, 3 Rue de Seichamps, CS 71040 Pulnoy, 54272 Essey-lès-Nancy Cedex, France; E-Mails: [email protected] (O.F.); [email protected] (M.-D.V.-D.); [email protected] (G.Pé.); [email protected] (G.Pa.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +33-247-052-316; Fax: +33-610-786-695. Received: 14 March 2014; in revised form: 31 July 2014 / Accepted: 1 August 2014 / Published: 20 August 2014 Abstract: The aim of this study was to evaluate the influence of the depth of the dermis on the measured collagen and elastin levels and to establish the correlation between the amount of these two extracellular matrix (ECM) components and age. Multiphoton Microscopy (MPM) that measures the autofluorescence (AF) and second harmonic generation (SHG) was used to quantify the levels of elastin and collagen and to determine the SAAID (SHG-to-AF Aging Index of Dermis) at two different skin depths. A 50 MHz ultrasound scanner was used for the calculation of the Sub Epidermal Non Echogenic Band (SENEB). -

Blood Vitronectin Is a Major Activator of LIF and IL-6 in the Brain Through Integrin–FAK and Upar Signaling Matthew P
© 2018. Published by The Company of Biologists Ltd | Journal of Cell Science (2018) 131, jcs202580. doi:10.1242/jcs.202580 RESEARCH ARTICLE Blood vitronectin is a major activator of LIF and IL-6 in the brain through integrin–FAK and uPAR signaling Matthew P. Keasey1, Cuihong Jia1, Lylyan F. Pimentel1,2, Richard R. Sante1, Chiharu Lovins1 and Theo Hagg1,* ABSTRACT Microglia and astrocytes express the VTN receptors αvβ3 and α β We defined how blood-derived vitronectin (VTN) rapidly and potently v 5 integrin (Herrera-Molina et al., 2012; Kang et al., 2008; activates leukemia inhibitory factor (LIF) and pro-inflammatory Milner, 2009; Welser-Alves et al., 2011). Microglia and astrocytes, interleukin 6 (IL-6) in vitro and after vascular injury in the brain. as well as endothelial cells, are major producers of pro- α in vitro Treatment with VTN (but not fibrinogen, fibronectin, laminin-111 or inflammatory cytokines, such as IL-6 and TNF , and collagen-I) substantially increased LIF and IL-6 within 4 h in after traumatic or ischemic injury to the brain (Banner et al., 1997; C6-astroglioma cells, while VTN−/− mouse plasma was less effective Erta et al., 2012; Lau and Yu, 2001) or upon self-induction by IL-6 than that from wild-type mice. LIF and IL-6 were induced by (Van Wagoner and Benveniste, 1999). IL-6 is a major regulator of a intracerebral injection of recombinant human (rh)VTN in mice, but variety of inflammatory disorders and a target for therapies (Hunter induction seen upon intracerebral hemorrhage was less in VTN−/− and Jones, 2015). -

With Caviar, Keratin & Collagen
WITH CAVIAR, KERATIN & COLLAGEN Professional treatments for colour, bleaching, care and maintenance with CAVIAR, KERATIN and COLLAGEN Professional styling products with CAVIAR, KERATIN and COLLAGEN Technical professional products with CAVIAR, KERATIN and COLLAGEN 2 Professional products by Very high technology professional products to colour, treat and protect hair from the continuous chemical and environmental stress caused on a daily basis. Formulas based on: Caviar Keratin Collagen 3 WITH CAVIAR, KERATIN & COLLAGEN Colouring permanentCream professional ammonia PPD • Respects the hair structure thanks to an exposure free free time of 12 minutes • Non-progressive • Ammonia free • Paraphenylenediamine free • Unleashes all the EFFICANCY of its active principles and maximum COLOURING POWER Mix 1 : 1 4 WITH CAVIAR, KERATIN & COLLAGEN 1. Respect for scalp and hair thanks to a shorter processing time 2. Maximum grey hair coverage 3. Lightens up to 4 tones 4. Ammonia free and Paraphenylenediamine free 5. Safe application even on customers with a sensitive scalp 6. Extreme colour brilliancy and uniformity 7. Very easy and practical to use 8. Long lasting reflections 9. High colour fastness 10. Great protection action 11. Effective restructuring action benefits 12. Maximum conditioning 5 WITH CAVIAR, KERATIN & COLLAGEN Benefits 1 2 3 Respect for scalp and hair thanks to a shorter processing Lightens up to Maximum grey hair 4 tones time coverage 6 WITH CAVIAR, KERATIN & COLLAGEN has been developed according Cosmetic colour pDT BASE Be Colour 12 Minute Dpe DIAMINOPHENOXYETHANOL to the rules of the “molar stoichiometry,” a technique creamy gel with RESORCINOL m-AMINOPHENOL of colouring clear and uncompromising. It is based on CAVIAR, a mathematical principle according to which the molar concentration of the dye base is equal to the molar KERATIN and concentration of the sum of the other colouring couplers. -

Therapeutic Drug Class
BUREAU FOR MEDICAL SERVICES WEST VIRGINIA MEDICAID EFFECTIVE PREFERRED DRUG LIST WITH PRIOR AUTHORIZATION CRITERIA 04/01/11 This is not an all-inclusive list of available covered drugs and includes only Version 2011.9 managed categories. Refer to cover page for complete list of rules governing this PDL. • Prior authorization for a non-preferred agent in any category will be given only if there has been a trial of the preferred brand/generic equivalent or preferred formulation of the active ingredient, at a therapeutic dose, that resulted in a partial response with a documented intolerance. • Prior authorization of a non-preferred isomer, pro-drug, or metabolite will be considered with a trial of a preferred parent drug of the same chemical entity, at a therapeutic dose, that resulted in a partial response with documented intolerance or a previous trial and therapy failure, at a therapeutic dose, with a preferred drug of a different chemical entity indicated to treat the submitted diagnosis. (The required trial may be overridden when documented evidence is provided that the use of these preferred agent(s) would be medically contraindicated.) • Unless otherwise specified, the listing of a particular brand or generic name includes all legend forms of that drug. OTC drugs are not covered unless specified. • PA criteria for non-preferred agents apply in addition to general Drug Utilization Review policy that is in effect for the entire pharmacy program, including, but not limited to, appropriate dosing, duplication of therapy, etc. • The use of pharmaceutical samples will not be considered when evaluating the members’ medical condition or prior prescription history for drugs that require prior authorization. -
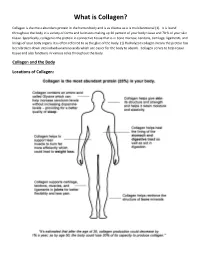
What Is Collagen?
What is Collagen? Collagen is the most abundant protein in the human body and is as diverse as it is multifunctional (1). It is found throughout the body in a variety of forms and functions making up 30 percent of your body tissue and 70 % of your skin tissue. Specifically, collagen is the protein in connective tissue that is in bone marrow, tendons, cartilage, ligaments, and linings of your body organs. It is often referred to as the glue of the body. (1) Hydrolyzed collagen means the protein has been broken down into individual amino acids which are easier for the body to absorb. Collagen serves to help repair tissue and also functions in various roles throughout the body. Collagen and the Body Locations of Collagen: What Does Collagen Do? Collagen has numerous structural properties but also plays a vital role in the repair of almost all the body’s tissues. Some diseases are directly linked to lacking this essential protein. Depending on which part of the body it is located, collagen serves different purposes. In skin: Found in the inner layer, this connective tissue gives the skin its structure and strength and also functions in the replacement of dead skin cells. A lack of collagen in the skin can contribute to a decrease in skin health leading to stretch marks, dark spots, and infections as well as affecting the skin’s ability to maintain moisture. In internal organs and blood vessels: In the lining of your organs like in the stomach, kidneys, blood vessels and spleen, collagen functions as a protective covering and a fibrous barrier. -

Probing Prothrombin Structure by Limited Proteolysis Laura Acquasaliente, Leslie A
www.nature.com/scientificreports OPEN Probing prothrombin structure by limited proteolysis Laura Acquasaliente, Leslie A. Pelc & Enrico Di Cera Prothrombin, or coagulation factor II, is a multidomain zymogen precursor of thrombin that undergoes Received: 29 November 2018 an allosteric equilibrium between two alternative conformations, open and closed, that react diferently Accepted: 2 April 2019 with the physiological activator prothrombinase. Specifcally, the dominant closed form promotes Published: xx xx xxxx cleavage at R320 and initiates activation along the meizothrombin pathway, whilst the open form promotes cleavage at R271 and initiates activation along the alternative prethrombin-2 pathway. Here we report how key structural features of prothrombin can be monitored by limited proteolysis with chymotrypsin that attacks W468 in the fexible autolysis loop of the protease domain in the open but not the closed form. Perturbation of prothrombin by selective removal of its constituent Gla domain, kringles and linkers reveals their long-range communication and supports a scenario where stabilization of the open form switches the pathway of activation from meizothrombin to prethrombin-2. We also identify R296 in the A chain of the protease domain as a critical link between the allosteric open-closed equilibrium and exposure of the sites of cleavage at R271 and R320. These fndings reveal important new details on the molecular basis of prothrombin function. Te response of the body to vascular injury entails activation of a cascade of proteolytic events where zymo- gens are converted into active proteases1. In the penultimate step of this cascade, the zymogen prothrombin is converted to the active protease thrombin in a reaction catalyzed by the prothrombinase complex composed of the enzyme factor Xa, cofactor Va, Ca2+ and phospholipids. -

Insights Into Vitamin K-Dependent Carboxylation: Home Field Advantage Francis Ayombil 1 and Rodney M
Editorials 15. Iyer S, Uren RT, Dengler MA, et al. Robust autoactivation for apop - guide clinical decision making in acute myeloid leukemia: a pilot tosis by BAK but not BAX highlights BAK as an important therapeu - study. Leuk Res. 2018;64:34-41. tic target. Cell Death Dis. 2020;11(4):268. 18. Zelenetz AD, Salles G, Mason KD, et al. Venetoclax plus R- or G- 16. Matulis SM, Gupta VA, Neri P, et al. Functional profiling of veneto - CHOP in non-Hodgkin lymphoma: results from the CAVALLI phase clax sensitivity can predict clinical response in multiple myeloma. 1b trial. Blood. 2019;133(18):1964-1976. Leukemia. 2019;33(5):1291-1296. 19. Adams CM, Clark-Garvey S, Porcu P, Eischen CM. Targeting the 17. Swords RT, Azzam D, Al-Ali H, et al. Ex-vivo sensitivity profiling to BCL2 family in B cell lymphoma. Front Oncol. 2019;8:636. Insights into vitamin K-dependent carboxylation: home field advantage Francis Ayombil 1 and Rodney M. Camire 1,2 1Division of Hematology and the Raymond G. Perelman Center for Cellular and Molecular Therapeutics, The Children’s Hospital of Philadelphia and 2Department of Pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA E-mail: RODNEY M. CAMIRE - [email protected] doi:10.3324/haematol.2020.253690 itamin K-dependent (VKD) proteins play critical recognized that the propeptide sequence is critical for VKD roles in blood coagulation, bone metabolism, and protein carboxylation. 6 This insight led to the development Vother physiologic processes. These proteins under - of GGCX substrates that contained a propeptide sequence go a specific post-translational modification called and portions of the Gla domain which are superior when 7,8 gamma ( γ)-carboxylation which is critical to their biologic compared to FLEEL alone. -

Empowering Collagen Targeting for Diagnosis and Treatment of Human Conditions
EmpoweringEmpowering collagen collagen targetingtargeting for for diagnosis prognosis of fibrotic and treatment of Manka,SW. 2012 conditions.human conditions. 1 3Helix as a platform diagnostic company 2020 2022 2030 Innovative research reagent for Clinic histopathology providing Platform fibrotic prognostic detection of collagen damage best in class prognostic ability in within multi-disease states liver fibrosis (NAFLD, NASH) Strengthening IP portfolio with market Clinical histopathology AND Non-Invasive approaches serum testing and medical disrupting products while subsequently Analytic specific reagent to allow for fast imaging developing strong partnerships with world market access leading companies for commercialization Targeting impactful markets of IPF, kidney Focused on paired biopsy research and fibrosis, AMD, Keloids and cardiac fibrosis collaboration with clinical laboratories. in addition to fibrotic liver diseases. 2 Liver Fibrosis market is GROWING Fatty Liver Fibrotic Liver Healthy Liver Cirrhosis NAFLD NASH USA Epidemiology 328 Million 83 Million 16 Million 1.5 Million (2015) Predicted USA Epidemiology 360 Million 101 Million 27 Million 3.4 Million (2030) Estes,C. 2018 3 NASH Therapeutics are finishing clinical trials and are coming to market. • VK2809 Phase II • OCALIVA (OCA) • NDA Filed • $78,000/year current cost • $20,000 predicted • Resmetirom Phase III • 23% respond to treatment 16 Million NASH patients in the USA * $20,000 • $320 Billion Annual Cost for treatable market Aramchol Phase III/IV 4 Stratification of patient population is needed to reduce unnecessary therapeutic intervention. • Progression of NAFL and 100% NASH is variable patient to NAFLD patient. 33% • Prediction of the progression can modify the Fibrosis Progression 20% disease intervention and treatment. Rapid Fibrosis • No product on the market Progression (stage 0 to stage today is equipped for 3/4 over 5.9 years) prognosis of liver fibrosis Singh, S.