Physiochemical Characteristics of Groundwater and Its Suitability For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NAAC Self Study Report 2015
NAAC Self Study Report 2015 DHARMAPURAM GNANAMBIGAI GOVERNMENT ARTS COLLEGE FOR WOMEN MAYILADUTHURAI – 609001 NAGAPATTINAM DISTRICT TAMILNADU Track ID: TNCOGN 10047 Website: www.dggacollege.edu.in E-mail ID: [email protected] INSTITUTIONAL ACCREDITATION CYCLE 3 SELF STUDY REPORT FOR AFFILIATED COLLEGES Submitted to National Assessment and Accreditation Council P.O. Box.No. 1075, Nagarbhavi, Bangaluru – 560 072 MAY 2015 D.G.G.A.C (W), MAYILADUTHURAI. Page i NAAC Self Study Report 2015 INDEX PAGE SECTION CONTENT NO. A DECLARATION i B CERTIFICATE OF COMPLIANCE ii BHARATHIDASAN UNIVERSITY C iii AFFILIATION CERTIFICATE UNIVERSITY GRANTS COMMISSION 2(f) D v & 12(B) CERTIFICATE E PREFACE vi F VISION, MISSION AND OBJECTIVES vii G EXECUTIVE SUMMARY viii H SWOC - ANALYSIS xiv I NAAC-INSTITUTIONAL STRUCTURE xvi J PROFILE OF THE COLLEGE 21 CRITERIA WISE ANALYTICAL REPORT 1. Curricular Aspects 2. Teaching Learning and Evaluation 3. Research, Consultancy and Extension 4. Infrastructure and Learning K 33 Resources 5. Students Support and Progression 6. Governance, Leadership and Management 7. Innovations L POST ACCREDITATION INITIATIVES 133 EVALUATIVE REPORTS OF THE M 141 DEPARTMENTS ANNEXURE Annexure I N Annexure II 246 Annexure III Annexure IV D.G.G.A.C (W), MAYILADUTHURAI. Page ii NAAC Self Study Report 2015 SECTION C: BHARATHIDASAN UNIVERSITY AFFILIATION CERTIFICATE : D.G.G.A.C (W), MAYILADUTHURAI. Page iii NAAC Self Study Report 2015 D.G.G.A.C (W), MAYILADUTHURAI. Page iv NAAC Self Study Report 2015 SECTION D: UNIVERSITY GRANTS COMMISSION 2(f) & 12(B) CERTIFICATE : D.G.G.A.C (W), MAYILADUTHURAI. Page v NAAC Self Study Report 2015 Section E Dharmapuram Gnanambigai Government Arts College for Women’s Self Study Report for the third cycle of accreditation by NAAC is an epoch-making milestone in its endeavour towards higher education objectives. -
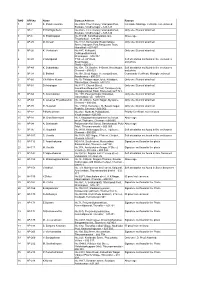
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -

Public Works Department Irrigation
PUBLIC WORKS DEPARTMENT IRRIGATION Demand No - 40 N.T.P. SUPPLIED BY THE DEPARTMENT PRINTED AT GOVERNMENT CENTRAL PRESS, CHENNAI - 600 079. POLICY NOTE 2015 - 2016 O. PANNEERSELVAM MINISTER FOR FINANCE AND PUBLIC WORKS © Government of Tamil Nadu 2015 INDEX Sl. No. Subject Page 3.4. Dam Rehabilitation and 41 Sl. No. Subject Page Improvement Project 1.0. 1 (DRIP) 1.1.Introduction 1 4.0. Achievements on 45 Irrigation Infrastructure 1.2. 2 During Last Four Years 1.3. Surface Water Potential 4 4.1. Inter-Linking of Rivers in 54 1.4. Ground Water Potential 5 the State 1.5. Organisation 5 4.2. Artificial Recharge 63 Arrangement Structures 2.0. Historic Achievements 24 4.3. New Anicuts and 72 3.0. Memorable 27 Regulators Achievements 4.4. Formation of New Tanks 74 3.1. Schemes inaugurated by 27 / Ponds the Hon’ble Chief 4.5. Formation of New 76 Minister through video Canals / Supply conferencing on Channels 08.06.2015 4.6. Formation of New Check 81 3.2. Tamil Nadu Water 31 dams / Bed dams / Resources Consolidation Grade walls Project (TNWRCP) 4.7. Rehabilitation of Anicuts 104 3.3. Irrigated Agriculture 40 4.8. Rehabilitation of 113 Modernisation and Regulators Water-bodies Restoration and 4.9. Rehabilitation of canals 119 Management and supply channels (IAMWARM) Project Sl. No. Subject Page Sl. No. Subject Page 4.10. Renovation of Tanks 131 5.0. Road Map for Vision 200 4.11. Flood Protection Works 144 2023 4.12. Coastal Protection 153 5.1. Vision Document for 201 Works Tamil Nadu 2023 4.13. -
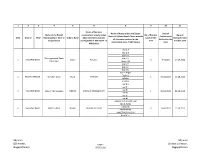
Containment Zone List As on 03.05.2020.Xlsx
1234 5 6 8 91011 Name of the Zone Name of Areas within the Zones- Date of Name of the Block/ containment activity taken No of Positive Date of Name of Village/Area ( Please provide Containment S.NO District HUD Municipality or Zone in Urban/ Rural place (Sector/ Location/ Cases in the testing for Last all the areas involved in the Initiated in the Corporatrion Zone) (within 5 KM Active + 2 area Positive Case contaiment zone- 7 KM radius) Area KM Buffer) Ward-7 Ward-8 Ward-10 Tharangambadi Town Ward-10 1 NAGAPATINAM Urban Poraiyar 3 9.4.2020 14.04.2020 Panchayat Ward -10 Ward-11 Ward-14 Ward-15 Kasim Nagar Thaikkal 2 NAGAPATTINAM Kollidam Block Rural PUTHUR 1 07.04.2020 11.04.2020 Kollidam Erukkur ward 2 ward 4 ward 5 3 NAGAPATINAM Sirkazhi Municipality URBAN SIRKAZHI MUNICIPALITY 1 06-04-2020 02.04.2020 ward 6 ward 7 ward 8 AGARA PERUNTHOTTAM MAIN ROAD EMBAVAI 4 NAGAPATINAM Sirkazhi Block RURAL PERUNTHOTTAM4 16/4/2020 11.04.2020 AGRAHARAM AMBETHKER NAGAR BEACH ST, Sd/-xxxx Sd/-xxxx DD Health , Page 1 District Collector, Nagapattinam 05-05-2020 Nagapattinam Name of the Zone Name of Areas within the Zones- Date of Name of the Block/ containment activity taken No of Positive Date of Name of Village/Area ( Please provide Containment S.NO District HUD Municipality or Zone in Urban/ Rural place (Sector/ Location/ Cases in the testing for Last all the areas involved in the Initiated in the Corporatrion Zone) (within 5 KM Active + 2 area Positive Case contaiment zone- 7 KM radius) Area KM Buffer) WARD 1 WARD 2 WARD 3 WARD 3 WARD WARD WARD 3 WARD -

Officials from the State of Tamil Nadu Trained by NIDM During the Year 209-10 to 2014-15
Officials from the state of Tamil Nadu trained by NIDM during the year 209-10 to 2014-15 S.No. Name Designation & Address City & State Department 1 Shri G. Sivakumar Superintending National Highways 260 / N Jawaharlal Nehru Salai, Chennai, Tamil Engineer, Roads & Jaynagar - Arumbakkam, Chennai - 600166, Tamil Nadu Bridges Nadu, Ph. : 044-24751123 (O), 044-26154947 (R), 9443345414 (M) 2 Dr. N. Cithirai Regional Joint Director Animal Husbandry Department, Government of Tamil Chennai, Tamil (AH), Animal husbandry Nadu, Chennai, Tamil Nadu, Ph. : 044-27665287 (O), Nadu 044-23612710 (R), 9445001133 (M) 3 Shri Maheswar Dayal SSP, Police Superintending of Police, Nagapattinam District, Tamil Nagapattinam, Nadu, Ph. : 04365-242888 (O), 04365-248777 (R), Tamil Nadu 9868959868 (M), 04365-242999 (Fax), Email : [email protected] 4 Dr. P. Gunasekaran Joint Director, Animal Animal Husbandary, Thiruvaruru, Tamil Nadu, Ph. : Thiruvarur, Tamil husbandary 04366-205946 (O), 9445001125 (M), 04366-205946 Nadu (Fax) 5 Shri S. Rajendran Dy. Director of Department of Agriculture, O/o Joint Director of Ramanakapuram, Agriculture, Agriculture Agriculture, Ramanakapuram (Disa), Tamil Nadu, Ph. : Tamil Nadu 04567-230387 (O), 04566-225389 (R), 9894387255 (M) 6 Shri R. Nanda Kumar Dy. Director of Statistics, Department of Economics & Statistics, DMS Chennai, Tamil Economics & Statistics Compound, Thenampet, Chennai - 600006, Tamil Nadu Nadu, Ph. : 044-24327001 (O), 044-22230032 (R), 9865548578 (M), 044-24341929 (Fax), Email : [email protected] 7 Shri U. Perumal Executive Engineer, Corporation of Chennai, Rippon Building, Chennai - Chennai, Tamil Municipal Corporation 600003, Ph. : 044-25361225 (O), 044-65687366 (R), Nadu 9444009009 (M), Email : [email protected] National Institute of Disaster Management (NIDM) Trainee Database is available at http://nidm.gov.in/trainee2.asp 58 Officials from the state of Tamil Nadu trained by NIDM during the year 209-10 to 2014-15 8 Shri M. -

National Agricultural Development Project – District Agriculture Plan
Wrapper Project Team Foreword Preface Executive Summary Chapter I Chapter II Chapter III Chapter IV Chapter V Chapter VI Table of Contents Annexure NATIONAL AGRICULTURAL DEVELOPMENT PROGRAMME (NADP) DISTRICT AGRICULTURE PLAN NAGAPATTINAM DISTRICT Centre for Agricultural and Rural Development Studies (CARDS) Tamil Nadu Agricultural University Coimbatore – 641 003 2008 NATIONAL AGRICULTURAL DEVELOPMENT PROJECT – DISTRICT AGRICULTURE PLAN PROJECT TEAM Overall Coordination : Dr.K.Palanisami, Director, CARDS and Nodal Officer (NADP) Dr.R.Venkatram, Professor and Principal Coordinator (NADP) District Level Coordination : Mrs.V.M.Indumathi, Assistant Professor, Dept.of ARM, TNAU Dr.R.Rajendran, Professor, KVK, Sikkal Dr.K.C.Gowthaman, Professor, KVK, Sikkal Mr.T.Manivannan, Joint Director of Agriculture Mr.S.Subbaiyah, Agricultural Officer Mr.Ilangovan, Deputy Director of Horticulture Mr.D.Napolean, Executive Engineer Mr.S.Madhanogopal, Assistant Engineer Tamil Nadu Agricultural University Prof. C.RAMASAMY COIMBATORE-641 003 Vice-Chancellor TAMIL NADU INDIA. FOREWORD Date ........................... The National Development Council resolved that Agricultural Development strategies must be reoriented to meet the needs of farmers and called upon the Central and State governments to evolve a strategy to rejuvenate agriculture with a commitment to achieve four per cent annual growth in the agricultural sector during the 11th plan. The council also recommended special Additional Central Assistance Scheme named National Agriculture Development Programme (NADP) be launched. To implement this, formulation of District level action plans is the pre-requisite and thus District Agriculture Plan of various districts in Tamil Nadu has been prepared with the financial assistance of Government of India. The task of preparing the District Agriculture Plan has been given to Tamil Nadu Agricultural University by Government of Tamil Nadu. -

Nagapatinam District : Environmental Profile
NAGAPATINAM DISTRICT : ENVIRONMENTAL PROFILE In this chapter, the basic facts of the study area have been given with the view to have area awareness about the study area. 3.1 Geographical Location of the District The Nagapattinam district lies on the east coast to the south of Cuddalore district and another part of the Nagapattinam district lies to the south of Karaikkal and Tiruvarur districts. Its northern boundary is about 75 Km southwards from the Head Quarters of the Cuddalore district. Thanjavur district and Tiruvarur district flank it on the west and on the south and east it is bordered by the Bay of Bengal. The district lies between 10.25and 11.40North Longitude and 7649and 80.01East longitude. The general geological formation of the district is plain and coastal. The Cauvery and its offshoots are the principal rivers. Rising in the Coorg Mountains, this river bifurcates about nine miles at the west of Trichy into two branches, of which the northern one takes the name of Coleroen and the southern one retains that of the Cauvery. All along the course of Cauvery and its dis-tributaries, on both the banks numerous narrow strips of river porombokes lands called Paduagais that are cut up by countless patta lands. These strips ranging in width from about 3 metres to 100 meters are made up of bits of lands. Even though the Padugais and Poromboke lands are very dry they are very fertile. Flood banks of Cauvery and its dis-tributaries are away from the watercourses in the upper reaches here the river is wide and closer in the lower reaches and the river become narrow gradually. -

World Bank Document
PROCUREMENT PLAN Project information: India; Tamil Nadu Irrigated Agriculture Modernization Project; P158522 Project Implementation agency: The lead implementing agency will be the WRD under the administrative jurisdiction of Principal Secretary, PWD. Other implementing agencies will be the Public Disclosure Authorized Departments of Agriculture, Agricultural Engineering, Agricultural Marketing and Agribusiness, Horticulture, Animal Husbandry and Fisheries; Tamil Nadu Agricultural University (TNAU), Tamil Nadu Fisheries University (TNFU), and Tamil Nadu Veterinary and Animal Sciences University (TANUVAS). A project implementation cell (PIC) will be established in each of participating line department and agency to oversee the implementation of their specific activities. The key functions of each PIC will be to prepare, implement, monitor their annual work plans and coordinate with MDPU. The PIC will consist of a nodal officer, and other staff in technical, procurement, finance, and safeguards areas as needed. Date of the Procurement Plan: Dec 14, 2016 Period covered by this Procurement Plan: 18 months Public Disclosure Authorized Preamble In accordance with paragraph 5.9 of the “World Bank Procurement Regulations for IPF Borrowers” (July 2016) (“Procurement Regulations”) the Bank’s Systematic Tracking and Exchanges in Procurement (STEP) system will be used to prepare, clear and update Procurement Plans and conduct all procurement transactions for the Project. This textual part along with the Procurement Plan tables in STEP constitute the Procurement Plan for the Project. The following conditions apply to all procurement activities in the Procurement Plan. The other elements of the Procurement Plan as required under paragraph 4.4 of the Public Disclosure Authorized Procurement Regulations are set forth in STEP. -

Tamil Nadu Siddha Medical Council, Chennai - 106
TAMIL NADU SIDDHA MEDICAL COUNCIL, CHENNAI - 106. REGISTER OF 2D BARCODE ISSUED REGISTERED SIDDHA MEDICAL PRACTITIONERS UPTO 31st MARCH 2017 (Ref No. 60/TNSMC/2017.) A - Class No. VI(1)/330/2017. Sl.No. Reg. No. Name Father's Name Qualification Date of Regn. Address (1) (2) (3) (4) (5) (6) (7) ARIYALUR DISTRICT 1 4608 Dr. AISHWARYA A K. Ambalavanan B.S.M.S. 22/06/2015 No.1334, Palani Andavar Koil Street, Vilandai, Andimadam, Ariyalur - 621801 2 3316 Dr. AKHILA DEVI S P S. Panneer Selvam B.S.M.S. 20/05/2009 I-26-A, Anumar Koil Street, T.Palur, Ariyalur - 612904 M.D.(S) 21/07/2016 3 3993 Dr. AKILA K R. KALIYAPERUMAL B.S.M.S. 24/05/2012 No.1/89, North Street, Sirukalathur Post, Sendurai Taluk, M.D.(S) 14/03/2016 Ariyalur - 621710 4 4693 Dr. AMALA M V. Manickam B.S.M.S. 20/07/2015 No.3/759-3/154, West Street, Mathumadakki, Keezhamaligai Post, Sendurai Taluk, Ariyalur - 621710 5 3903 Dr. ANANTHI K V K. Vaithianathan B.S.M.S. 13/07/2011 No.112, Main Road, Variyankaval, Udayarpalayam Taluk, 2 M.D.(S) 27/01/2015 Ariyalur - 621806 6 4137 Dr. ANBARASI A S. Anbalagan B.S.M.S. 22/07/2013 No.123, Ponparappi / Kudikadu Post, Sendurai Taluk, M.D.(S) 14/03/2017 Ariyalur - 621710 7 4841 Dr. ANILADEVI P S. PANNEER SELVAM B.S.M.S. 07/01/2016 No.1/26, Anumar Koil Street, T. Palur, Ariyalur - 612904 8 4399 Dr. ANITHA A M. -

Madurai, Thiruvarur, Salem, Thanjavur
Initial Environmental Examination Document Stage: Project Number: 40648-037 February 2018 IND: Infrastructure Development Investment Program for Tourism (IDIPT) Tranche 4 — Development and Improvement of Infrastructure Facilities at (a) Arulmigu Kallalagar Thirukoil, Alagarkoil, Melur Taluk, Madurai District; (b) Arulmigu Abathsagayeswarar Temple at Alangudi Village, Thiruvarur District; (c) Arulmigu Kumaragiri Dhandayudhabani Swamy Thirukkoil at Ammapet in Salem District; (d) Arulmigu Kailasanathar Swamy Temple at Thingalur Village in Thanjavur District; (e) Naganatha Swamy Temple at Thirunageswarm, Thanjavur District; (f) Sri Swetharanyeswarar Temple at Thiruvenkadu, Nagapattinam District; (g) Veerapur, Tiruchirappalli District; (h) Construction of Pilgrims rest house in Arulmigu Naganathaswamy Temple, Keelaperumpallam, Nagapattinam District Package No. IDIPT/TN/T4/NCB/08/2017 Prepared by the Department of Tourism and Culture Government of Tamil Nadu for the Asian Development Bank. This initial environmental examination report is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. CURRENCY EQUIVALENTS (as of 15 December -

Nagapattinam - an Introduction 2
Table of Contents S.No Contents Page No 1. Nagapattinam - An Introduction 2. District Diagnostic Study 3. Socio Demographic Profile of the District 3.1 Population 3.2 Sex ratio 3.3 Literacy 3.4 SC,ST population 3.5 Occupation Profile 3.6 Community Based Organisations 3.7 Farmer Producer Organisations 4.0 Geographical Features 4.1 Topography 4.2 Land use pattern 4.3 Climate and rainfall 4.4 Soil 4.5 Water resources 5.0 Status of Groundwater 6.0 District Infrastructure 6.1 Electricity 6.2 Factory accommodation 6.3 Railways 6.4 Roads 6.5 Sea Port 6.6 Post & Telegraph 6.7 Banking and Financial Institutions 6.8 Training facilities 6.9 Regulated Markets 6.10 Tamilnadu Civil supplies Corporation storage points 7.0 Farm Sector 7.1 Land holding pattern 7.2 Irrigation 7.3 Cropping pattern 7.3.1 Area and Production major crops 7.3.2 Other crops 7.3.3 Horticulture and Plantation crops 7.4 Blockwise Major Crop Cultivation 8 Resource Institutions 9.0 Allied sectors 9.1 Livestock and Poultry 9.2 Fisheries 10.0 Non farm sector 10.1 Industrial scenario in the district 10.2 Small Scale Industries 10.3 Medium and Large Scale Industries 10.4 MSME clusters 10.5 Salt pan – Vedaranyam 11.0 Heritage sites - Rural Tourism 12.0 Credit and Insurance 13.0 Potential Activities in Nagapattinam District 13.1 Commodity Prioritization 14.0 Qualitative Skill gap 15.0 Conclusion List of tables Table 1 : List of Village Panchayats in TNRTP Blocks of Nagapattinam District Table 2: Population details of Nagapattinam District Table 3. -

World Bank Document
PROCUREMENT PLAN Project information: India; Tamil Nadu Irrigated Agriculture Modernization Project; P158522 Project Implementation agency: The lead implementing agency will be the WRD under the administrative jurisdiction of Principal Secretary, PWD. Other implementing agencies will be the Public Disclosure Authorized Departments of Agriculture, Agricultural Engineering, Agricultural Marketing and Agribusiness, Horticulture, Animal Husbandry and Fisheries; Tamil Nadu Agricultural University (TNAU), Tamil Nadu Fisheries University (TNFU), and Tamil Nadu Veterinary and Animal Sciences University (TANUVAS). A project implementation cell (PIC) will be established in each of participating line department and agency to oversee the implementation of their specific activities. The key functions of each PIC will be to prepare, implement, monitor their annual work plans and coordinate with MDPU. The PIC will consist of a nodal officer, and other staff in technical, procurement, finance, and safeguards areas as needed. Date of the Procurement Plan: Dec 14, 2016 Period covered by this Procurement Plan: 18 months Public Disclosure Authorized Preamble In accordance with paragraph 5.9 of the “World Bank Procurement Regulations for IPF Borrowers” (July 2016) (“Procurement Regulations”) the Bank’s Systematic Tracking and Exchanges in Procurement (STEP) system will be used to prepare, clear and update Procurement Plans and conduct all procurement transactions for the Project. This textual part along with the Procurement Plan tables in STEP constitute the Procurement Plan for the Project. The following conditions apply to all procurement activities in the Procurement Plan. The other elements of the Procurement Plan as required under paragraph 4.4 of the Public Disclosure Authorized Procurement Regulations are set forth in STEP.