VET 8(1) 14 Anupama Sharma.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Franchisee Area
FRANCHISEES DATA (Punjab LSA) UPDATED Upto 31.03.2021 S.No. Name of Franchisee (M/s) Address SSA Type (Distributor/ Area/ Wheather Area of Operation of territory Wheather through Contact Person Contact no Email id Type of Services retailer/ own outlet) Terrtiory/ through EOI/ EOI/ Migration/ (Prepaid/ Franchisee Zone Code Migration/ Lookafter Postpaid/ Both) Lookafter 1 EOI AJNALA (AJNALA U, Migrated js.milanagencies@gm Both Opp. AB palace, Near Radha Swami Milan Agencies Amritsar Franchisee ASR-01 BACHIWIND, BEHARWAL, Jasjeet Sethi 9464777770 ail.com Dera, Ram Tirath Road,Amritsar BHILOWAL, CHAMIARI, 2 EOI Albert Road, BATALA Road, Migrated js.milanagencies@gm Both Milan Agencies 39,the mall Amritsar Amritsar Franchisee ASR-02 Majitha Road-II, RANJIT Jasjeet Sethi 947803575 ail.com AVENUE, JAIL ROAD, VERKA 3 Migrated City (Katra Sher Singh, Tarn Migrated js.uscomputers@gmai Both U.S.Comp.Products 77, Hall Bazar, Amritsar Amritsar Franchisee ASR-03 Taran Road-II, Bhagtanwala) Jasjeet Sethi 9463393947 l.com 4 EOI JANDIALA (AKALGARH,BAL Migrated chanienterprises@yo Both 12,Baba Budha ji Avenue, G.T.Road, Avtar Singh Chani Enterprises Amritsar Franchisee ASR-04 KHURD,BHANGALI KALAN, 9417553366 ur.com Amritsar Chani BUNDALA, 5 EOI Tarn Taran (BATH, BUCHAR Migrated chanienterprises@yo Both Avtar Singh Chani Enterprises Tur Market, Tarn taran Amritsar Franchisee ASR-05 KHURD, CHABAL, CHEEMA 9530553536 ur.com Chani KHURD, DABURJI, DHAND 6 EOI PATTI (ALGON KOTHI, Migrated js.uscomputers@gmai Both Near amardeep palace old Kairon road U.S.Comp.Products Amritsar Franchisee ASR-06 AMARKOT, BASARKE, Jasjeet Sethi 9465272988 l.com ,Patti BHANGALA, BHIKHIWIND U, 7 Empowered RAYYA (BABA BAKALA, BAGGA, New abhinavsharma007@ Both VPO Rayya ,Tehsil Baba Bakala,Distt C.K. -
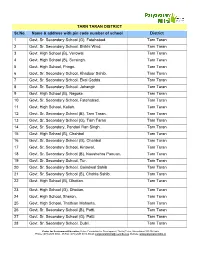
TARN TARAN DISTRICT Sr.No. Name & Address With
TARN TARAN DISTRICT Sr.No. Name & address with pin code number of school District 1 Govt. Sr. Secondary School (G), Fatehabad. Tarn Taran 2 Govt. Sr. Secondary School, Bhikhi Wind. Tarn Taran 3 Govt. High School (B), Verowal. Tarn Taran 4 Govt. High School (B), Sursingh. Tarn Taran 5 Govt. High School, Pringri. Tarn Taran 6 Govt. Sr. Secondary School, Khadoor Sahib. Tarn Taran 7 Govt. Sr. Secondary School, Ekal Gadda. Tarn Taran 8 Govt. Sr. Secondary School, Jahangir Tarn Taran 9 Govt. High School (B), Nagoke. Tarn Taran 10 Govt. Sr. Secondary School, Fatehabad. Tarn Taran 11 Govt. High School, Kallah. Tarn Taran 12 Govt. Sr. Secondary School (B), Tarn Taran. Tarn Taran 13 Govt. Sr. Secondary School (G), Tarn Taran Tarn Taran 14 Govt. Sr. Secondary, Pandori Ran Singh. Tarn Taran 15 Govt. High School (B), Chahbal Tarn Taran 16 Govt. Sr. Secondary School (G), Chahbal Tarn Taran 17 Govt. Sr. Secondary School, Kirtowal. Tarn Taran 18 Govt. Sr. Secondary School (B), Naushehra Panuan. Tarn Taran 19 Govt. Sr. Secondary School, Tur. Tarn Taran 20 Govt. Sr. Secondary School, Goindwal Sahib Tarn Taran 21 Govt. Sr. Secondary School (B), Chohla Sahib. Tarn Taran 22 Govt. High School (B), Dhotian. Tarn Taran 23 Govt. High School (G), Dhotian. Tarn Taran 24 Govt. High School, Sheron. Tarn Taran 25 Govt. High School, Thathian Mahanta. Tarn Taran 26 Govt. Sr. Secondary School (B), Patti. Tarn Taran 27 Govt. Sr. Secondary School (G), Patti. Tarn Taran 28 Govt. Sr. Secondary School, Dubli. Tarn Taran Centre for Environment Education, Nehru Foundation for Development, Thaltej Tekra, Ahmedabad 380 054 India Phone: (079) 2685 8002 - 05 Fax: (079) 2685 8010, Email: [email protected], Website: www.paryavaranmitra.in 29 Govt. -

CONGRESSIONAL RECORD— Extensions of Remarks E1596 HON
E1596 CONGRESSIONAL RECORD Ð Extensions of Remarks August 7, 1998 occupation and denial of human rights. As a lege. As a Marine during WWII, he saw action every day, other innocent people will join the nation, we must insist that turkey withdraw its in the South Pacific. He is survived by his wife ranks of the disappeared.'' With nuclear weap- occupying forces and allow the return of refu- Beverly, a daughter Kate; two sons: Vaughn ons involved in South Asia, these terrible vio- gees to their communities. and Arch; and five grandchildren. lations of basic human rights are even more We must send a clear message stating that f dangerous to the entire world. violations of human rights and international KHALISTANI DELEGATION law will not be tolerated, especially when per- I am inserting Dr. Aulakh's testimony and TESTIFIES AT UNITED NATIONS petrated by a nation to which we grant signifi- the Council of Khalistan's press release into cant amounts of foreign aid. A truly democratic the RECORD for the information of my col- foreign policy will seek the restoration of a HON. DAN BURTON leagues. I urge them to read it carefully. It is united Greek-Cypriot state and serve as a tes- OF INDIANA frightening, but quite informative. Thank you, IN THE HOUSE OF REPRESENTATIVES tament to our commitment to democratic self- Mr. Speaker. Thursday, August 6, 1998 government and fundamental freedoms. TESTIMONY OF DR. GURMIT SINGH AULAKH, f Mr. BURTON of Indiana. Mr. Speaker, re- PRESIDENT, COUNCIL OF KHALISTAN BEFORE cently a delegation of Khalistani Americans led A TRIBUTE TO IAN B. -

Present: Appellant: Absent Respondent: Sh. Parvinder (JE)
PUNJAB STATE INFORMATION COMMISSION Red Cross Building, Near Rose Garden,Sector 16, Chandigarh. Ph: 0172-2864112, Email: - [email protected] Visit us: - www.infocommpunjab.com, Helpline No.0172-2864100 Sh. Davinder Pal Singh, (9316433434) S/o Sh. Gurdip Singh, Booth No 38 FF, Chotti Baradari Shopping Complex, Patiala-147001. ………….Appellant/Complainant Versus Public Information Officer ……………………………Respondent O/o Municipal Corporation, Patiala. First Appellate Authority O/o Commissioner, Municipal Corporation, Patiala. Appeal Case No.354 of 2021 Present: Appellant: Absent Respondent: Sh. Parvinder (J.E) 9888471616 ORDER 1. The Appellant/Complainant filed appeal/complaint case in the Commission dated 07.01.2021. Accordingly, the case is fixed for today. 2. Written Submission by Appellant: A letter dated 04.03.2021 vide diary no. 5039 is received by the bench of undersigned vide which the appellant Sh. Davinder Pal Singh has acknowledged that that the sought information has been provided to him and is satisfied with the same. This email is taken on record. 3. As the information stands supplied therefore, no cause of action is required in this case. Hence, the instant appeal case is disposed & closed. Sd/- Chandigarh (Maninder Singh Patti) Dated: 10.03.2021 State Information Commission PUNJAB STATE INFORMATION COMMISSION Red Cross Building, Near Rose Garden,Sector 16, Chandigarh. Ph: 0172-2864112, Email: - [email protected] Visit us: - www.infocommpunjab.com, Helpline No.0172-2864100 Sh. Ishant Sharma, (8699333971) s/o Sh. Surinder Sharma, Resident of Shop No 7, Second Floor, ………….Appellant/Complainant Hardev Complex, Sehdev Market, Jalandhar. Versus Public Information Officer O/o Estate Officer, ……………………………Respondent Jalandhar Development Authority, Jalandhar. -

Sr. No. License No Name of Travel Agent Office Name Office Address
Sr. License Name of Travel Office Name Office Address License Type Till which No. No Agent Date Licence is Valid 1 1 Navdeep Gupta Sharma Travels, Old Court Sharma Travels, Old Court Consultancy 28.09.2022 Road,Moga Road,Moga 2 2 Navdeep Gupta Sharma Travels, Old Court Sharma Travels, Old Court Ticketing 28.09.2022 Road,Moga Road,Moga 3 3 Gurmilap Singh Micro Global, Amolak Bhawan, G.T. Micro Global, Amolak Bhawan, G.T. Travel Agency 28.09.2022 Road, Moga Road, Moga 4 4 Gurmilap Singh Micro Global, Amolak Bhawan, G.T. Micro Global, Amolak Bhawan, G.T. Coaching Institute 28.09.2022 Road, Moga Road, Moga of Ielts 5 5 Gurmilap Singh Micro Global, Amolak Bhawan, G.T. Micro Global, Amolak Bhawan, G.T. Consultancy 28.09.2022 Road, Moga Road, Moga 6 6 Gurmilap Singh Micro Global, Amolak Bhawan, G.T. Micro Global, Amolak Bhawan, G.T. Ticketing 28.09.2022 Road, Moga Road, Moga 7 7 Kuldeep Singh Rai Universal Visa Hub, Green Velly, Universal Visa Hub, Green Velly, Travel Agency 28.09.2022 Dosanj Road, Moga Dosanj Road, Moga 8 8 Kuldeep Singh Rai Universal Visa Hub, Green Velly, Universal Visa Hub, Green Velly, Coaching Institute 28.09.2022 Dosanj Road, Moga Dosanj Road, Moga of Ielts 9 9 Kuldeep Singh Rai Universal Visa Hub, Green Velly, Universal Visa Hub, Green Velly, Consultancy 28.09.2022 Dosanj Road, Moga Dosanj Road, Moga 10 10 Kuldeep Singh Rai Universal Visa Hub, Green Velly, Universal Visa Hub, Green Velly, Ticketing 28.09.2022 Dosanj Road, Moga Dosanj Road, Moga 11 11 Deepak Manchanda Go Global Consultancy, Sub Jail Go Global Consultancy, -

Complaint Case No. 541 of 2018
PUNJAB STATE INFORMATION COMMISSION Red Cross Building, Madhya Marg, Sector 16, Chandigarh Ph. No. 0172-2864111 FAX. No. - 0172-2864125 E-mail – [email protected] Visit us - www.infocommpunjab.com Harpal Singh Sodhi S/o Sh. Dasondha Singh Sodhi, House No.15, Kalgidhar Enclave, Baltana, Zirakpur, District Mohali (Punjab) ……. Complainant Vs Public Information Officer, O/o The Principal Secretary to Government of Punjab, Department of Finance, Punjab Civil Secretariat-1, Chandigarh ..…Respondent Complaint Case No. 541 of 2018 Present : None on behalf of the applicant. None on behalf of the respondent. ORDER This case was last heard on 21.11.2018. After examining the documents placed on record, it is found that the respondent PIO has sent a reply vide letter no. 1058 dated 05.12.2018 signed by Superintendent-cum-PIO, Department of Finance ( Finance Personnel – 1 Branch), which has been received in the Commission vide Diary No. 24655 dated 07.12.2018, showing that the requisite information has already been supplied to the applicant, Sh. Harpal Singh Sodhi. It is taken on record. The applicant, Sh. Harpal Singh Sodhi, through a letter dated 16.12.2018, which has been received in the Commission through an e-mail, has intimated the Commission that deficiencies have already been pointed out to the PIO and the PIO has not removed the same till date. It is taken on record. An opportunity is given to the respondent PIO to remove the deficiencies, pointed out by the applicant, by the next date of hearing. th The case is adjourned to 14 January, 2019 (Monday) at 11:00 A. -

Disaster Management Plan (District Patiala) 2020-2021
DISASTER MANAGEMENT PLAN (DISTRICT PATIALA) 2020-2021 Kumar Amit , IAS Deputy Commissioner-cum-Chairperson District Disaster Management Authority Patial Index Sr. No. Subject Page 1. Physical Features. 1 2. Physiographics. 2-3 3. Maps 4-6 4. Flow Chart of Activities Regarding activities done during 7 floods 5. Important Phone No. of Flood Control Rooms/ NDRF Team / 8 Armed Forces Contacts 6 Important Phone/Mobile No. at District Level 9-10 7 Police Department at District Level Contacts 11 8 Drainage Department / / PSPCL Contacts 12-13 9 Senior Medical Officer's Contact/Animal Husbandry Patiala 14 10 Trained Homegurads/Tained Boat Driver in Distt Patiala 15 Contacts 11 Important Phone/Mobile No. at Sub Division Patiala 16 12 Flood prone area/Relief centres in Sub Division Patiala 17-29 13 Important Phone/Mobile No. at Sub Division Rajpua 30-31 14 Flood prone area/Relief centres in Sub Division Rajpura 32-35 15 Important Phone/Mobile No. at Sub Division Dhudhan 36 Sadhan 16 Flood prone area/Relief centres in Sub Division Dhudhan 37-41 Sadhan 17 Important Phone/Mobile No. at Sub Division Nabha. 42 18 Flood prone area/Relief centres in Sub Division Nabha 43-44 19 Important Phone/Mobile No. at Sub Division Samana 45-46 20 Flood prone area/Relief centres in Sub Division Samana 47 21 Important Phone/Mobile No. at Sub Division Patran 48 22 Flood prone area/Relief centres in Sub Division Patran 49-52 23 Flood relief Material at Different Sub Divisions. 52 24 Non Government Organisations/NGO, Sub Division Wise. 53-57 25 Guidelines on Minimum Standards of Relief 58-63 1.Physical Features Origin of Name: The district derives its name from the district headquarters town of Patiala, which is said to have been founded about 1762 AD; by Baba Ala Singh, the founder of Patiala State. -

Senior Advocates of Punjab & Haryana High Court, Chandigarh
Senior Advocates of Punjab & Haryana High Court, Chandigarh Upto 16.4.2012 Sr.No Name of Counsel Address with Phone No. Enrolment Office Order No. & (Sarv Shri/Smt.) No. date 1. Sh. Ashok Resi, # 149, Sector 11-A, Chandigarh, - O/O No. 34 LB Aggarwal, SSC. Ph.No. 0172-2746998, 2747561, 3(14) SSC PSPCL (AG Pb.), 0172-2740287, Fax: 0172-2747074 Dated Pb. & Haryana High 9.11..2011 Court Chd. 2. Balbir Singh Wasu Bangalow No.1119, P-7/1957 143 dt. 23.8.2002 Sector 8-C, Chandigarh Ph. 2544240, 93160-42712 3. Chattar Bhujh 215-A, Sector-6, Panchkula P-253/63 143 dt. 23.8.2002 Kaushik Ph. R-2560803 O-2745091 4. Roshan Lal Sharma 558, Sector 10-D, Chandigarh P-106/65 143 dt. 23.8.2002 Ph. 94172-55983 2741206 5. J.P.S. Sandhu 1197, Sector 44-B, Chandigarh P-136/70 143 dt. 23.8.2002 Ph. 2624147, 98141-05385 6. Vijay Pal Singh 853, Sector -7, Panchkula Since4 1977 143 dt. 23.8.2002 Ph. 2597853 7. Chander Mohan 556, Sector 8-B, Chandigarh P-176/76 143 dt. 23.8.2002 Sharma Ph. 2780755, 2778038 8. S.S. Virk 2661-C, MIG (S), 26 years 143 dt. 23.8.2002 Sector 70, Mohali. Ph. 2265093 (R) 2743347 (H.C), 94170-33093 9. Manohar Kumar 5593, Sector-38, P-294/78 143 dt. 23.8.2002 Garg (West) Chandigarh Ph. 2625163 98140-55593 10. J.S. Yadav 98/4 WDL, Panchkula P-114/81 143 dt. 23.8.2002 Ph. -

Sr. No. Person-Name MHL-UNQ-ID/#Address Details
Sr. Quartitine Quartitine Person-Name MHL-UNQ-ID/#Address Details No. Start-Date End-Date 1 AAKASH SHETTY MHL-10837/HOUSE NO. 502, B-5, PURAV PREMIUM APARTMENT, SECTOR-88, MOHALI 03-Jun-20 17-Jun-20 2 ABHIJIT BHATNAGAR MHL-10833/# 1003, FALCON VIEW, BLOCK H, SECTOR-66A, MOHALI 03-Jun-20 17-Jun-20 3 ABHISHEK TYAGI MHL-9277/DERABASSI 03-Jun-20 17-Jun-20 4 ADESH MHL-9261/DERABASSI 03-Jun-20 17-Jun-20 5 ADHAR AGGARWAL MHL-9373/240 GULMOHAR CITY DERABASSI 03-Jun-20 17-Jun-20 6 ADHAR AGGARWAL MHL-10804/240/B10 - GULMOHAR CITY EXTENSION, HAIBATPUR ROAD,DERABASSI MOHALI 03-Jun-20 17-Jun-20 7 AHMAD, BASIT MHL-10845/73 FF PRIME CITY KHARAR 03-Jun-20 17-Jun-20 8 AJAY MHL-9288/DERABASSI 03-Jun-20 17-Jun-20 9 AJAY KUMAR MHL-9290/SAIDPURA 03-Jun-20 17-Jun-20 10 AKHLISHWAR JAISWAL MHL-10834/220, MOTIA ROYAL FAME, SECTOR-117, MOHALI 03-Jun-20 17-Jun-20 AKSHAANT MR 11 MHL-10861/#602 METRO TOWER PEER MUCHALLA, ZIRAKPUR 03-Jun-20 17-Jun-20 SHARMA 12 ALAKJOT KAUR MHL-9228/SECRTOR 23 CHANDIGARH 03-Jun-20 17-Jun-20 13 AMANPREET MHL-9308/267, PH 6, MOHALI 03-Jun-20 17-Jun-20 14 AMANPREET SINGH MHL-9320/2643, SEC 79, MOHALI 03-Jun-20 17-Jun-20 15 AMAR SINGH MHL-10822/62C BELLA HOME DERA BASSI 03-Jun-20 17-Jun-20 16 AMARJIT KAUR MHL-10777/3427, SEC 71, MOHALI 03-Jun-20 17-Jun-20 17 AMIT MR INANI MHL-10853/E 817 ESCON ARENA ZIRAKPUR MOHALI PUNJAB 03-Jun-20 17-Jun-20 18 ANIL KUMAR MHL-9391/NO 03-Jun-20 17-Jun-20 19 ANITA MHL-9244/2230 SEC 15 PANCHKULA 03-Jun-20 17-Jun-20 20 ANITA SHARMA MHL-9331/DH MOHALI 03-Jun-20 17-Jun-20 21 ANJALI MHL-9271/BHANKAPRUR 03-Jun-20 -

Punjab Medical Council Electoral Rolls Upto 31-01-2018
Punjab Medical Council Electoral Rolls upto 31-01-2018 4678. Dr. Sanjeev Kumar s/o M.B.B.S. 1999 Ward No.7, Shahid Bhagat Singh Nagar, 10-5-99 31004 05.02.2019 Sh. Shiv Dayal M.D.(TB & Res.Dis.) Sujanpur Distt. Pathankot 2005 4679. Dr. Brijendra Gupta S/o M.B.B.S. 1999 7B, Dashmesh Enclave, Loharh Road, 10.05.99 31006 6.8.2019 Sh. T.R. Gupta Dip.inOrtho. 2008 Zirakpur Distt. Mohali 4680. Dr.Neetu Kuka r D /O M.B.B.S. 1999 H.No.73, Medical Campus, Faridkot 3100710- 5-99 16.05.2020 Sh. Jass Raj Kukar M.D.(Path) 2008 10.05.99 4681. Dr. Somnath Chanchalram M.B.B.S. 1990 D-Block, Mount Avenue, Near Ex M.C. 14-5-99 31013 4.11.2022 Mahe S/o Sh. Chanchalram M.D.(Gen.Med.) House, Hoshairpur Mahe 1993 4682. Dr. Sukhjit Kaur d/o M.B.B.S. 1999 Grewal Hospital & Nursing Home, 14-5-99 31014 03.05.2019 Sh. Ujjagar Singh Grewal Chowk,Malerkotla, Distt. Sangrur. 4683. Dr. Navneet Dhaliwal D/o M.B.B.S. 1998 # 657, Phase VI, Sector 56, Mohali. 27-5-99 31017 24.05.2021 Sh. Gurnam Singh Dhaliwal 4684. Dr. Sukhwinder Singh s/o M.B.B.S. 1999 208, Capt H.P.S., Dyami Nagar, Mitha 11-6-99 31021 05.04.2019 Pur Road, Jalandhar. Sh. Mohinder Singh 4685. Dr. Jagjit Kaur D/o M.B.B.S 1998 VPO Massanian, Trhsil Batala Punjab), 21-06-99 31025 05.09.2020 Sh. -
LIST of TELEPHONE NOS. of POLICE OFFICERS PPCR PH.NO.2740058, 2740298, EPBX-2704, FAX NO.2740901, Control Room Sec/Wing-2740418 & 2741699-F
LIST OF TELEPHONE NOS. OF POLICE OFFICERS PPCR PH.NO.2740058, 2740298, EPBX-2704, FAX NO.2740901, Control Room Sec/Wing-2740418 & 2741699-F. P.P.HQ Sector 9,Chg Exchange 2748100 to 2748106, Cyber Crime Control Room SAS Nagar 0172-2220042,Control Room Prisons 0172-2703460,85579-20000,Community Policing Phase -7, SAS Nagar-2220050 NRI Control Room 2260042-43 NRI (website:-www.nripunjab.gov.in),PPHC office at Phase-7, SAS NAGAR PBX No. 0172-4080111- 113 & 4080107-Fax. (INT,CID/HQRs, SAS-N, 0172-2293500,9147,Fax-9131) (28.09.2021) NAME OF THE OFFICER DESIGNATION PBX OFFICE RESIDENCE MOBILE Sh. Iqbal Preet Singh DGP/Punjab, HOPF 2402 2743272, 2743772, Sahota, IPS 2403 2741832-F, Sh. Dinkar Gupta, IPS DGP - - Sh. S.Chattopadhyaya, IPS DGP/PSPCL, PB.Chg. 0172-2745321,2216929-F 0175-2213010 Sh. M.K. Tiwari, IPS DGP-cum Chairman-cum-MD/PPHC, 2225057(SAS-N) 4080107-F Phase-7,SAS-N Sh. V.K.Bhawra, IPS DGP/Punjab Home Guards & Dir. - 2701353 (2701169 CR), Civil Defence Chandigarh 2701149-F Sh. Prabodh Kumar, IPS Special DGP Prov. Pb. 2323 2920501 Sh. Rohit Chaudhary, IPS Special DGP/PSHR Comm. 2635446,2635435-F (SCO No.20-22 Sec.34,Chg) Sh.Sanjeev Kumar Kalra, IPS Special DGP/Railways Patiala 2521 0172-2749739,0175-2212038 Sh. B.K.Uppal, IPS Chief Director, State 2218111 Vigilance Bureau,Pb.SAS-N Sh.Sharad Satya Chauhan,IPS ADGP/Traffic (Dir. Lead Agency 2222 2745222 on Road Safety, Transport Pb.) Sh.Harpreet Singh Sidhu,IPS ADGP/STF 9201 2219036 Sh.Gaurav Yadav, IPS ADGP/Law & Order (Addl. -
IPS Officers on State to State Deputation to Other States
IPS Officers on Deputation to Central Govt./Other Organizations (As on 17.05.2021) Sr. Name with source Year of Present Posting Date from Date of No. of recruitment and allotment which on completion of rank in the State deputation deputation S/Sh./Smt. period 1. S.K.Goel, IPS (RR) RR:1984 Director, (RA&W), Cabinet 05.03.2001 This officer is Sectt., Govt. of India, New on permanent Delhi. secondment basis. 2. Parag Jain, IPS, (RR) RR:1989 Addl. Secy., Cabinet Sectt., 06.07.2010 On Permanent Govt of India, New Delhi Secondment. (since .7.2019). 3. Kapil Dev, IPS (RR) RR:1995 Director, Cabinet Sectt., 17.11.2008 On Permanent Govt. of India, New Delhi. Secondment. 4. Rajiv Ahir, IPS (RR) RR:1996 Jt. Director, IB Hqrs., MHA, (in Int. New Delhi since 01.05.18. Bureau from 23.02.09 to 05.12.14) 5. Pavan Kumar Rai, RR:1997 Jt. Secy., Cabinet Sectt., 01.07.2010 On Permanent IPS (RR) Govt. of India, New Delhi. Secondment. 6. Nilabh Kishore, IPS RR:1998 IG/Northern FTR Hqrs., 15.02.2018 14.02.2023 (RR) ITBP, Seemadwar, Dehradun since 26.04.2019. 7. Dinesh Pratap Singh, RR:2000 Director, Cabinet Sectt., 01.07.2014 On Permanent IPS (RR) GOI, New Delhi. Secondment. 8. Ashish Choudhary, RR:2003 DIG/National Investigation 04.07.2018 IPS (RR) Agency, New Delhi. 9. Harsh Kumar Bansal RR:2005 Deputy Director/ 06.07.2015 05.07.2023 (RR) Intelligence Bureau, GOI, New Delhi. 10. Dhanpreet Kaur (RR) RR:2006 Dy. Secy., Information 31.05.2016 30.05.2023 and Broadcasting, Govt.