Interferon Lambda: Modulating Immunity in Infectious Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polymorphism Rs368234815 of Interferon Lambda 4 Gene and Spontaneous Clearance of Hepatitis C Virus in Haemodialysis Patients: a Case Control Study
Polymorphism rs368234815 of Interferon Lambda 4 Gene and Spontaneous Clearance of Hepatitis C Virus in Haemodialysis Patients: A Case Control Study Alicja Grzegorzewska ( [email protected] ) Poznań University of Medical Sciences https://orcid.org/0000-0001-8087-4696 Adrianna Mostowska Uniwersytet Medyczny imienia Karola Marcinkowskiego w Poznaniu Monika Świderska Uniwersytet Medyczny imienia Karola Marcinkowskiego w Poznaniu Wojciech Marcinkowski Fresenius NephroCare Polska Sp zoo Ireneusz Stolarek Polska Akademia Nauk Marek Figlerowicz Polska Akademia Nauk Paweł P. Jagodziński Uniwersytet Medyczny imienia Karola Marcinkowskiego w Poznaniu Research article Keywords: haemodialysis, hepatitis C virus, interferon-λ4 gene, spontaneous viral clearance, transcription factors Posted Date: December 16th, 2019 DOI: https://doi.org/10.21203/rs.2.18966/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published on January 22nd, 2021. See the published version at https://doi.org/10.1186/s12879-021-05777-6. Page 1/16 Abstract Background In non-uremic subjects, IFNL4 rs368234815 predicts the HCV clearance. We investigated whether rs368234815 is associated with spontaneous HCV clearance in haemodialysis patients and whether it is a stronger predictor of HCV resolution than the IFNL polymorphisms already associated with HCV clearance in haemodialysis subjects. An association of rs368234815 with survival and alterations in transcription factor binding sites (TFBS) caused by IFNL polymorphisms were also evaluated. Methods Among 161 haemodialysis patients with positive anti-HCV antibodies, 68 (42.2%) spontaneously resolved HCV infection, whereas 93 remained HCV RNA positive. Patients were tested for near IFNL3 rs12980275, IFNL3 rs4803217, IFNL4 rs12979860, IFNL4 rs368234815, and near IFNL4 rs8099917. -

Interleukin 28 Is a Potential Therapeutic Target for Sepsis
Clinical Immunology 205 (2019) 29–34 Contents lists available at ScienceDirect Clinical Immunology journal homepage: www.elsevier.com/locate/yclim Interleukin 28 is a potential therapeutic target for sepsis T ⁎ Qin Luoa,b, Yi Liuc, Shuang Liub, Yibing Yinb, Banglao Xud, Ju Caoa, a Department of Laboratory Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China b Key Laboratory of Diagnostic Medicine designated by the Ministry of Education, Chongqing Medical University, Chongqing, China c Department of Intensive Care Unit, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China d Department of Laboratory Medicine, Guangzhou First People's Hospital, School of Medicine, South China University of Technology, Guangzhou, Guangdong, China ARTICLE INFO ABSTRACT Keywords: Identification of new therapeutic targets for the treatment of sepsis is imperative. We report here that cytokine Interleukin-28 IL-28 (IFN-λ) levels were elevated in clinical and experimental sepsis. Neutralization of IL-28 protected mice Sepsis from lethal sepsis induced by cecal ligation and puncture (CLP), which was associated with improved bacterial Infection clearance and enhanced neutrophil infiltration. Conversely, administration of recombinant IL-28 aggravated Immunity mortality, facilitated bacterial dissimilation and limited neutrophil recruitment, in the model of sepsis induced Neutrophil by CLP. This study defines IL-28 as a detrimental mediator during sepsis and identifies a potential therapeutic target for the immune therapy in sepsis. 1. Introduction immunopathology of sepsis is still poorly understood. To address this issue, we examined the potential role of IL-28 in the Each year, about 31.5 million individuals develop sepsis, and up to progression of sepsis. -

Mechanism of Action Through an IFN Type I-Independent Responses To
Downloaded from http://www.jimmunol.org/ by guest on September 25, 2021 is online at: average * The Journal of Immunology , 12 of which you can access for free at: 2012; 188:3088-3098; Prepublished online 20 from submission to initial decision 4 weeks from acceptance to publication February 2012; doi: 10.4049/jimmunol.1101764 http://www.jimmunol.org/content/188/7/3088 MF59 and Pam3CSK4 Boost Adaptive Responses to Influenza Subunit Vaccine through an IFN Type I-Independent Mechanism of Action Elena Caproni, Elaine Tritto, Mario Cortese, Alessandro Muzzi, Flaviana Mosca, Elisabetta Monaci, Barbara Baudner, Anja Seubert and Ennio De Gregorio J Immunol cites 33 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription http://www.jimmunol.org/content/suppl/2012/02/21/jimmunol.110176 4.DC1 This article http://www.jimmunol.org/content/188/7/3088.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2012 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 -

MOUSE INTERLEUKIN-28B/INTERFERON-LAMBDA 3, CARRIER FREE Product Number: 12821-1 Lot Number: 5559 Size: 25 Μg
MOUSE INTERLEUKIN-28B/INTERFERON-LAMBDA 3, CARRIER FREE Product Number: 12821-1 Lot Number: 5559 Size: 25 µg Description: Mouse Interleukin-28B/Interferon-lambda 3, Carrier Free Source: A DNA sequence encoding the mature mouse IL-28B/IFN-λ3 (Asp 20 - Val 193) (Kotenko, S.V. et al., 2003, Nat. Immunol. 4(1):69 - 77) was expressed in E. coli. Form: Lyophilized Buffer: Phosphate-buffered saline (PBS) Reconstitution: It is recommended that sterile PBS be added to the vial to prepare a stock solution of no less than 100 μg/mL. Endotoxin: < 1 EU/µg Molecular Weight: The 175 amino acid residue methionyl form of recombinant mouse IL-28B has a predicted molecular mass of approximately 19.7 kDa. Purity: > 95% Synonyms: Mu IL-28B; Mu IFN-λ3 Accession #: NP_796370 Assays Used to Measure Bioactivity: Human HepG2 cells infected with encephalomyocarditis virus (Sheppard, P. et al., 2003, Nature Immunol. 4:63). The ED50 for this effect is typically 7.5 - 37.5 ng/mL. Shipping Conditions: Wet Ice Physical State of Product During Shipping: Lyophilized Special Conditions/Comments: After receipt, this product should be kept at -70˚C or below for retention of full activity. Upon reconstitution, this cytokine can be stored under sterile conditions at 2˚C to 8˚C for one month or at -20˚C to -70˚C in a manual defrost freezer for three months without detection loss of activity. Avoid repeated freeze-thaw cycles. For more information on protein handling, visit the PBL website at www.interferonsource.com . Product Information: Human IL-28A, IL-28B, and IL-29, also named interferon-λ2 (IFN-λ2), IFN-λ3, and IFN-λ1, respectively, are newly identified class II cytokine receptor ligands that are distantly related to members of the IL-10 family (11- 13% aa sequence identity) and type I IFN family (15 - 19% aa sequence identity).1 – 3 The genes encoding these three cytokines are localized to chromosome 19 and each is composed of multiple exons. -

The Role of Il-29 and Il-28B in the Innate Immune Response
THE ROLE OF IL-29 AND IL-28B IN THE INNATE IMMUNE RESPONSE Megumi A. Williamson A thesis submitted to the faculty of the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Master of Science in the Department of Periodontology, School of Dentistry Chapel Hill 2018 Approved by: Thiago Morelli Thiago Morelli Julie Marchesan Steven Offenbacher Antonio Amelio ©2018 Megumi A. Williamson ALL RIGHTS RESERVED ii ABSTRACT Megumi A. Williamson: The Role of IL-29 and IL-28B in the Innate Immune Response (Under the direction of Thiago Morelli, Julie Marchesan, Steven Offenbacher, and Antonio Amelio) Aims: Chronic periodontitis (CP) is an inflammatory disease induced by dysbiotic biofilm in a susceptible host, resulting in progressive attachment loss, and subsequent alveolar bone loss. Recent genome-wide association studies (GWAS) and genome-wide gene centric analysis on periodontal complex traits (PCTs) identified possible associations of IL-29 and IL- 28B with periodontal diseases. However, the underlying mechanisms for how these genes contribute to the pathogenesis of periodontitis are largely unknown. The aims of the present study were to explore the role of IL-29 and IL-28B and their gene polymorphisms in the innate immune response by dendritic cells. Materials and methods: To explore the effect of IL-29 on the cytokine production in response to TLR4 stimulation, the IL-29 gene was knocked-down in THP-1 cells using IL-29 shRNA lentiviral particles. Pro- and anti-inflammatory cytokine levels were measured with Luminex® multiplex assay. To assess the effect of genetic variations in IL- 29 and IL-28B, whole blood samples from fifteen subjects (6 subjects with major allele for both IL-29 and IL-28B, 5 subjects for major allele in IL-29 and minor allele in IL-28B, and 4 subjects with minor alleles for both genes) were collected and CD14+/CD16lo PBMCs were isolated to generate DCs. -

Health & Medicine Research
WEDNESDAY, MARCH 30, 2016 HEALTH & MEDICINE RESEARCH DAY HEALTH & MEDICINE RESEARCH DAY WEDNESDAY, MARCH 30, 2016 MARVIN CENTER MARVIN CENTER 800 21ST STREET, NW, 3RD FLOOR 800 21ST STREET, NW, 3RD FLOOR 8:00–9:00 a.m. Posters Setup (Grand and Continental Ballrooms) 12:00–2:00 p.m. Distribution of Box Lunches (MC 310) 12:30–3:00 p.m. Poster Presentations and Judging JACK MORTON AUDITORIUM (Grand and Continental Ballrooms) 805 21ST STREET, NW 3:00–4:00 p.m. Awards Ceremony and Oral Presentations (includes 10-minute presentations by winners of oral 8:00–9:00 a.m. Registration and Breakfast competition awards) (MC 309) 9:00–9:05 a.m. Welcome to Research Days 2016 Jeffrey S. Akman, MD Vice President for Health Affairs and Dean, MILKEN INSTITUTE SCHOOL OF School of Medicine and Health Sciences PUBLIC HEALTH BUILDING 9:05–9:10 a.m. Introduction of Keynote Address 950 NEW HAMPSHIRE AVENUE, NW, 1ST FLOOR AUDITORIUM Catherine Bollard, MD, FRACP, FRCPA Professor of Pediatrics and Microbiology, Immunology 4:30–4:35 p.m. Welcome Video & Introduction of Keynote Address and Tropical Medicine, School of Medicine and Health Sciences; Director, Program for Cell Lynn R. Goldman, MD, MS, MPH Enhancement and Technologies for Immunotherapy Michael and Lori Milken Dean, Milken Institute School (CETI), Children’s National Health System of Public Health; Professor of Environmental and Occupational Health 9:10–10:00 a.m. Keynote Address Kimberly Horn, EdD, MSW Stanley R. Riddell, MD Associate Dean for Research, Milken Institute School Fred Hutchinson Cancer Research Center, Clinical of Public Health; Professor of Prevention and Research Division; Professor of Oncology, University of Community Health Washington School of Medicine 4:35–5:15 p.m. -

Cytokines: from Basic Mechanisms of Control to New Therapeutics
This is a free sample of content from Cytokines: From Basic Mechanisms of Control to New Therapeutics. Click here for more information on how to buy the book. Index A Cryopyrin-associated periodic syndromes (CAPS), AKT 262–263 g chain family cytokine signaling, 9 CTCL. See Cutaneous T-cell lymphoma T-cell development role, 279 Cutaneous T-cell lymphoma (CTCL), interleukin-15 role, AMPs. See Antimicrobial peptides 448 Antimicrobial peptides (AMPs), 456 Cytokine release syndrome (CRS), interleukin-6 therapeutic Arid5a, 94 targeting, 93, 94 Atopic dermatitis Cytokine studies interleukin-17 studies, 130 agonist development, 458–459 interleukin-22 therapeutic targeting, 169 alarmins, 456–457 antagonist development, 459–460 assay development, 457 B definition, 455–456 B cell historical perspective, 456 g chain family cytokine function, 16–18 mimics, 457 interferon-g in activation, 226–227 mutation studies, 458 tumor necrosis factor in development and function, 105 signaling studies, 457–458 BCL11B, 280–281 b Common family cytokines. See also specific cytokines cancer studies, 46 D functions, 43–44 Daclizumab, 21 nervous system function, 46–47 DC. See Dendritic cell overview, 40–43 Dendritic cell (DC) receptors classical dendritic cells activation, 48–49, 51 cDC1 development and function, 397–398 assembly, 48 cDC2 development and function, 398–399 assembly, 48–51 GM-DCs, 403–404 interactions with other receptors, 47–48 human cell features, 401–402 proximal activation of signaling, 52–53 identification sepsis and inflammation studies, 44–45 mouse bone marrow, 396–397 therapy, 41–42, 53–56 surface markers for in vivo identification, 403 BLIMP1, 15 maturation, 404–406 Briakinumab, 151 Mo-DCs, 403 g chain family cytokine function, 19 C interferon-g in activation, 226 Candida albicans, interleukin-17 studies, 129 long noncoding RNA regulation of differentiation, CAPS. -

Molecular Characterization of Tea Catechin Treated Human Prostate Cancer Cell Lines Yewseok Suh
Florida State University Libraries Electronic Theses, Treatises and Dissertations The Graduate School 2006 Molecular Characterization of Tea Catechin Treated Human Prostate Cancer Cell Lines Yewseok Suh Follow this and additional works at the FSU Digital Library. For more information, please contact [email protected] THE FLORIDA STATE UNIVERSITY COLLEGE OF ARTS AND SCIENCES MOLECULAR CHARACTERIZATION OF TEA CATECHIN TREATED HUMAN PROSTATE CANCER CELL LINES By YEWSEOK SUH A Dissertation submitted to the Department of Chemistry and Biochemistry in partial fulfillment of the requirements for the degree of Doctor of Philosophy Degree Awarded: Summer Semester, 2006 The members of the Committee approve the dissertation of Yewseok Suh defended on May 12, 2006. Qing-Xiang Amy Sang Professor Directing Dissertation Thomas C.S. Keller III Outside Committee Member Joseph B. Schlenoff Committee Member Hong Li Committee Member Approved: Naresh Dalal, Chair, Department of Chemistry and Biochemistry Joseph Travis, Dean, College of Arts and Sciences The Office of Graduate Studies has verified and approved the above named committee members. ii This dissertation is dedicated to my parents for their endless love and encouragement, to my lovely wife Inok Park for her support, and to our charming daughter, Tae-won. iii ACKNOWLEDGEMENTS I would like to express my gratitude to my major professor for her support throughout the research. Also thanks to all my committee members, Dr. Thomas C. S. Keller III, Dr. Hong Li, and Dr. Joseph B. Schlenoff, for their support, advice, and guidance. Special thanks to our lab members especially, Ziad Sahab and Robert G. Newcomer and former lab member Douglas R. -

When a Good Interferon Goes Bad
JOURNAL OF INTERFERON & CYTOKINE RESEARCH Volume 00, Number 00, 2019 ª Mary Ann Liebert, Inc. DOI: 10.1089/jir.2019.0044 The IFN-l4 Conundrum: When a Good Interferon Goes Bad Olusegun O. Onabajo, Brian Muchmore, and Ludmila Prokunina-Olsson Since its discovery in 2013, interferon lambda 4 (IFN-l4) has received a reputation as a paradoxical type III IFN. Difficulties in detecting IFN-l4, especially in secreted form even led to questions about its existence. However, the genetic ability to generate IFN-l4, determined by the presence of the rs368234815-DG allele, is the strongest predictor of impaired clearance of hepatitis C virus (HCV) infection in humans. Significant modulation of IFN-l4 activity by a genetic variant (P70S) supports IFN-l4, and not other type III IFNs encoded in the same genomic locus, as the primary functional cause of the association with HCV clearance. Although the ability to produce IFN-l4 is associated with decreased HCV clearance, the recombinant IFN-l4 is active against HCV and other viruses. These observations present an apparent conundrum—when and how does a presumably good IFN, with anti-HCV activity, interfere with the ability to clear HCV? In this review, we discuss findings that suggest potential mechanisms for explaining this conundrum. Keywords: IFN-l4, type III interferon, SOCS1, USP18, regulation, HCV Introduction transiently overexpressed in vitro (Paquin and others 2016). The extent of conservation between these homologs suggests he family of class-2 cytokines includes type I in- an important functional role of IFN-l4 in evolution (Key and Tterferons (IFN-a, b, k, and o), type II IFN (IFN-g), type others 2014). -

2018 Chen Lingyan 1448129
This electronic thesis or dissertation has been downloaded from the King’s Research Portal at https://kclpure.kcl.ac.uk/portal/ Genetics and Epigenetics in Systemic Lupus Erythematosus Chen, Lingyan Awarding institution: King's College London The copyright of this thesis rests with the author and no quotation from it or information derived from it may be published without proper acknowledgement. END USER LICENCE AGREEMENT Unless another licence is stated on the immediately following page this work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence. https://creativecommons.org/licenses/by-nc-nd/4.0/ You are free to copy, distribute and transmit the work Under the following conditions: Attribution: You must attribute the work in the manner specified by the author (but not in any way that suggests that they endorse you or your use of the work). Non Commercial: You may not use this work for commercial purposes. No Derivative Works - You may not alter, transform, or build upon this work. Any of these conditions can be waived if you receive permission from the author. Your fair dealings and other rights are in no way affected by the above. Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 03. Oct. 2021 GENETICS AND EPIGENETICS IN SYSTEMIC LUPUS ERYTHEMATOSUS Lingyan CHEN Department of Medical & Molecular Genetics Faculty of Life Sciences & Medicine King’s College London This thesis is submitted for the degree of DoCtor of Philosophy (PhD) 1 To my parents 2 DeClaration The work described in this thesis was carried out within the Vyse Immunogenetics Group in the Department of Medical and Molecular Genetics at King’s College London under the supervision of Professor Timothy James Vyse and Dr David Lester Morris and between the years of October 2014 and March 2018. -
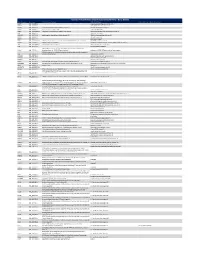
Ncounter® Autoimmune Discovery Consortium Panel
nCounter® Autoimmune Discovery Consortium Panel - Gene Details Official Symbol Accession Alias / Previous Symbol Official Full Name Other targets or Isoform Information AAMP NM_001087.3 angio associated migratory cell protein ABHD6 NM_020676.5 abhydrolase domain containing 6 ACKR2 NM_001296.3 CMKBR9,CCBP2;chemokine binding protein 2 atypical chemokine receptor 2 ACOXL NM_018308.1 acyl-Coenzyme A oxidase-like acyl-CoA oxidase like ACSL6 NM_001009185.1 FACL6;fatty-acid-Coenzyme A ligase, long-chain 6 acyl-CoA synthetase long chain family member 6 ADA NM_000022.2 adenosine deaminase ADAM30 NM_021794.2 a disintegrin and metalloproteinase domain 30 ADAM metallopeptidase domain 30 ADCY3 NM_004036.3 adenylate cyclase 3 ADCY7 NM_001114.4 adenylate cyclase 7 AFF3 NM_001025108.1 LAF4;lymphoid nuclear protein related to AF4,AF4/FMR2 family, member 3 AF4/FMR2 family member 3 AGAP2 NM_014770.3 CENTG1;centaurin, gamma 1 ArfGAP with GTPase domain, ankyrin repeat and PH domain 2 AHI1 NM_001134830.1 Abelson helper integration site Abelson helper integration site 1 AHR NM_001621.3 aryl hydrocarbon receptor AHSA2;AHA1, activator of heat shock 90kDa protein ATPase homolog 2 AHSA2 NM_152392.1 (yeast),activator of HSP90 ATPase homolog 2 activator of HSP90 ATPase homolog 2, pseudogene APECED;autoimmune regulator (autoimmune polyendocrinopathy candidiasis AIRE NM_000383.2 ectodermal dystrophy) autoimmune regulator AMIGO3 NM_198722.2 adhesion molecule with Ig like domain 3 ANKRD55 NM_024669.2 ankyrin repeat domain 55 ANTXR2 NM_058172.5 anthrax toxin receptor -

Supplementary Table 1
Supplementary Table 1. 492 genes are unique to 0 h post-heat timepoint. The name, p-value, fold change, location and family of each gene are indicated. Genes were filtered for an absolute value log2 ration 1.5 and a significance value of p ≤ 0.05. Symbol p-value Log Gene Name Location Family Ratio ABCA13 1.87E-02 3.292 ATP-binding cassette, sub-family unknown transporter A (ABC1), member 13 ABCB1 1.93E-02 −1.819 ATP-binding cassette, sub-family Plasma transporter B (MDR/TAP), member 1 Membrane ABCC3 2.83E-02 2.016 ATP-binding cassette, sub-family Plasma transporter C (CFTR/MRP), member 3 Membrane ABHD6 7.79E-03 −2.717 abhydrolase domain containing 6 Cytoplasm enzyme ACAT1 4.10E-02 3.009 acetyl-CoA acetyltransferase 1 Cytoplasm enzyme ACBD4 2.66E-03 1.722 acyl-CoA binding domain unknown other containing 4 ACSL5 1.86E-02 −2.876 acyl-CoA synthetase long-chain Cytoplasm enzyme family member 5 ADAM23 3.33E-02 −3.008 ADAM metallopeptidase domain Plasma peptidase 23 Membrane ADAM29 5.58E-03 3.463 ADAM metallopeptidase domain Plasma peptidase 29 Membrane ADAMTS17 2.67E-04 3.051 ADAM metallopeptidase with Extracellular other thrombospondin type 1 motif, 17 Space ADCYAP1R1 1.20E-02 1.848 adenylate cyclase activating Plasma G-protein polypeptide 1 (pituitary) receptor Membrane coupled type I receptor ADH6 (includes 4.02E-02 −1.845 alcohol dehydrogenase 6 (class Cytoplasm enzyme EG:130) V) AHSA2 1.54E-04 −1.6 AHA1, activator of heat shock unknown other 90kDa protein ATPase homolog 2 (yeast) AK5 3.32E-02 1.658 adenylate kinase 5 Cytoplasm kinase AK7