Chalcogenocarboxylic Acids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report 2013-2014
ANNUAL REPORT 2013 – 14 One Hundred and Fifth Year Indian Institute of Science Bangalore - 560 012 i ii Contents Page No Page No Preface 5.3 Departmental Seminars and IISc at a glance Colloquia 120 5.4 Visitors 120 1. The Institute 1-3 5.5 Faculty: Other Professional 1.1 Court 1 Services 121 1.2 Council 2 5.6 Outreach 121 1.3 Finance Committee 3 5.7 International Relations Cell 121 1.4 Senate 3 1.5 Faculties 3 6. Continuing Education 123-124 2. Staff 4-18 7. Sponsored Research, Scientific & 2.1 Listing 4 Industrial Consultancy 125-164 2.2 Changes 12 7.1 Centre for Sponsored Schemes 2.3 Awards/Distinctions 12 & Projects 125 7.2 Centre for Scientific & Industrial 3. Students 19-25 Consultancy 155 3.1 Admissions & On Roll 19 7.3 Intellectual Property Cell 162 3.2 SC/ST Students 19 7.4 Society for Innovation & 3.3 Scholarships/Fellowships 19 Development 163 3.4 Assistance Programme 19 7.5 Advanced Bio-residue Energy 3.5 Students Council 19 Technologies Society 164 3.6 Hostels 19 3.7 Award of Medals 19 8. Central Facilities 165-168 3.8 Placement 21 8.1 Infrastructure - Buildings 165 8.2 Activities 166 4. Research and Teaching 26-116 8.2.1 Official Language Unit 166 4.1 Research Highlights 26 8.2.2 SC/ST Cell 166 4.1.1 Biological Sciences 26 8.2.3 Counselling and Support Centre 167 4.1.2 Chemical Sciences 35 8.3 Women’s Cell 167 4.1.3 Electrical Sciences 46 8.4 Public Information Office 167 4.1.4 Mechanical Sciences 57 8.5 Alumni Association 167 4.1.5 Physical & Mathematical Sciences 75 8.6 Professional Societies 168 4.1.6 Centres under Director 91 4.2. -

Tables of Parameters Tables of Parameters for Extended Hückel Calculations Calculations
TABLES OF PARAMETERS FOR EXTENDED HÜCKEL CALCULATIONS Santiago Alvarez Universitat de Barcelona 1999 - 1 - PART 1. STANDARD Hii's and SLATER EXPONENTS AND COEFFICIENTS. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Rf Db Sg Bh Hs Mt La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Parameters for all elements except the shaded ones are included in these Tables. - 2 - General Observations References are to the original paper which first reported EH calculations with the given Hii for a related set of molecules or solids. For some elements, when no EH calculations have been reported, the experimental ionization potentials are given and the references correspond to those experimental values. Although these tables are intended to be of some use in locating atomic parameters, reference to the original work is encouraged. Ionization potentials ( Hii) a) All energies are in eV. Hii's for most transition metals are charge-iterated for model compounds like metal carbonyls or typical organometallic molecules with Cp, CH3, CO, etc. Exceptions, including bulk metals are conveniently indicated. For cases in which charge-iterated Hii's are not available, the standard sources are the Hinze and Jaffé's tables of ionization potentials.1 b) When alternative sets of Hii's were available for the same element, the one which fits better with the trend along a group or a period of the periodic table has been chosen. -

Chapter 1. Periodic Properties and Variation of Properties
Chapter 1. Periodic Properties and Variation of Properties PAGE NO : 16 Solution 1: Modern periodic law states that the physical and chemical properties of elements are a periodic function of their atomic numbers i.e., if the elements are arranged in the order of their atomic numbers, the elements with similar properties are repeated after definite regular intervals. Concept Insight: The elements are characterized by their atomic number as well as atomic weight. Modern periodic law uses atomic number which is number of protons or number of electrons present in an atom of an element. Solution 2: Modern periodic table consists of eighteen groups and seven periods. Concept Insight: Classification of elements on the basis of increasing atomic number is known as Modern Periodic Table. The vertical columns are called groups and the horizontal rows are called periods. Solution 3: The recurrence of similar properties of elements after certain regular intervals when they are arranged in the order of increasing atomic numbers is called periodicity. Concept Insight: Periodicity in properties is due to the repetition of similar outer electronic configuration of elements at certain regular intervals. Solution 4: In general, the elements belonging to a group have the same number of valence electrons .For example, all the group 1 elements have valency one since they have only one electron in their outermost shell. In general, the elements belonging to a period do not have same valency but their valence shell remains the same. For example, second period has 8 elements with atomic number 3 to 10 but in all of them the valence electrons are present in shell number two. -

Ditellurides
Molecules 2010, 15, 1466-1472; doi:10.3390/molecules15031466 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Novel Oxidative Ring Opening Reaction of 1H-Isotelluro- chromenes to Bis(o-formylstyryl) Ditellurides Haruki Sashida 1,*, Hirohito Satoh 1, Kazuo Ohyanagi 2 and Mamoru Kaname 1 1 Faculty of Pharmaceutical Sciences, Hokuriku University, Kanagawa-machi, Kanazawa 920-1181, Japan; E-Mail: [email protected] (M.K.) 2 Faculty of Pharmacy, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Kakuma-machi, Kanazawa 920-1192, Japan; E-Mail: [email protected] (K.O.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +81-76-229-6211; Fax: +81-76-229-2781. Received: 5 January 2010; in revised form: 2 February 2010 / Accepted: 5 March 2010 / Published: 9 March 2010 Abstract: The oxidation of 1-unsubstituted or 1-phenyl-1H-isotellurochromenes with m-chloroperbenzoic acid (mCPBA) in CHCl3 resulted in a ring opening reaction to produce as the sole products the corresponding o-formyl or benzoyl distyryl ditellurides, which were also produced by the hydrolysis of the 2-benzotelluropyrylium salts readily prepared from the parent isotellurochromene. Keywords: isotellurochromene; mCPBA oxidation; 2-benzotelluropyrylium salt; distyryl ditelluride 1. Introduction The preparation and investigation of the reactions of new tellurium-containing heterocycles have lately attracted increasing interest in the fields of both heterocyclic [1–3] and organotellurium chemistry [4–8]. We have been studying the syntheses and reactions of the novel tellurium- or selenium-containing heterocyclic compounds over the past twenty years. -

Combined Science Chemistry Booklet
COMBINED SCIENCE CHEMISTRY BOOKLET TASK: Complete the questions that your teacher sets on Show My Homework and use the mark scheme at the end of the document to check your responses. Topic 1 – Atomic Structure and the Periodic Table Q1. The periodic table on the Data Sheet may help you to answer these questions. (a) Part of the periodic table is shown below. The letters are not the symbols of the elements. Choose your answers only from the letters shown in the periodic table above. Which letter, A, B, C, D, E or F, represents (i) aluminium (1) Page 1 of 66 (ii) a Group 5 element (1) (iii) an alkali metal (1) (iv) the element with atomic (proton) number of 47 (1) (v) an element with seven electrons in its outer shell? (1) (b) The table shows the boiling points of the Group 7 elements. The elements are arranged in alphabetical order. Group 7 element Name Symbol Boiling point in °C Astatine At 337 Bromine 58 Chlorine Cl -34 Fluorine F -188 Iodine I 184 (i) The symbol for bromine is missing from the table. What is the symbol for bromine? Symbol = _______________ (1) (ii) Arrange these elements in order of decreasing boiling point. The first one and the last one have been done for you. At ______ ______ ______ F Highest boiling Lowest boiling point point Page 2 of 66 (1) (c) The table shows some statements about Group 7 elements. Tick ( ) the two correct statements. Tick ( ) They are halogens. They are metals. They become less reactive down Group 7. -

61Ni Synchrotron-Radiation-Based Mössbauer Absorption
Hyperfine Interact (2018) 239:11 https://doi.org/10.1007/s10751-018-1488-0 61Ni synchrotron-radiation-based Mossbauer¨ absorption spectroscopy of Ni nanoparticle composites Ryo Masuda1 · Hirokazu Kobayashi2,3 · Yoshimasa Aoyama2 · Makina Saito1 · Shinji Kitao1 · Hiroki Ishibashi1 · Shuichi Hosokawa1 · Takaya Mitsui4 · Yoshitaka Yoda5 · Hiroshi Kitagawa2 · Makoto Seto1,4 © Springer International Publishing AG, part of Springer Nature 2018 Abstract We obtained energy-domain 61Ni synchrotron-radiation-based Mossbauer¨ absorption spectra of three materials that relate to nanoparticles: Ni2(C8O6H2) metal- organic frameworks (MOFs), Ni nanoparticles synthesized by complete heat decomposition of the MOFs, and the composites of Ni nanoparticles and the MOFs synthesized by par- tial decomposition of the MOFs. The 61Ni abundance of all the samples was not enriched but we were successfully able to obtain their spectra in 1 day or less, by using a highly efficient measurement system where the internal conversion electrons from energy standard 61 Ni86V14 foil were detected. Although both nanoparticle constituent and MOF constituent in the composites included Ni atoms, the Mossbauer¨ parameters of the Ni nanoparticle con- stituent could be evaluated; the magnetic hyperfine field of the Ni nanoparticle constituent in the composites was different from that of the Ni nanoparticles obtained by the complete heat decomposition. This difference implied that the 3d and/or 4s electron configuration of the nanoparticle constituent were affected by the MOF constituent -

The Periodic Table: a Very Short Introduction VERY SHORT INTRODUCTIONS Are for Anyone Wanting a Stimulating and Accessible Way Into a New Subject
The Periodic Table: A Very Short Introduction VERY SHORT INTRODUCTIONS are for anyone wanting a stimulating and accessible way into a new subject. They are written by experts, and have been translated into more than 45 different languages. The series began in 1995, and now covers a wide variety of topics in every discipline. The VSI library currently contains over 600 volumes—a Very Short Introduction to everything from Psychology and Philosophy of Science to American History and Relativity—and continues to grow in every subject area. Very Short Introductions available now: ABOLITIONISM Richard S. Newman ACCOUNTING Christopher Nobes ADAM SMITH Christopher J. Berry ADOLESCENCE Peter K. Smith ADVERTISING Winston Fletcher AFRICAN AMERICAN RELIGION Eddie S. Glaude Jr AFRICAN HISTORY John Parker and Richard Rathbone AFRICAN POLITIC Ian Taylor AFRICAN RELIGIONS Jacob K. Olupona AGEING Nancy A. Pachana AGNOSTICISM Robin Le Poidevin AGRICULTURE Paul Brassley and Richard Soffe ALEXANDER THE GREAT Hugh Bowden ALGEBRA Peter M. Higgins AMERICAN CULTURAL HISTORY Eric Avila AMERICAN FOREIGN RELATIONS Andrew Preston AMERICAN HISTORY Paul S. Boyer AMERICAN IMMIGRATION David A. Gerber AMERICAN LEGAL HISTORY G. Edward White AMERICAN NAVAL HISTORY Craig L. Symonds AMERICAN POLITICAL HISTORY Donald Critchlow AMERICAN POLITICAL PARTIES AND ELECTIONS L. Sandy Maisel AMERICAN POLITICS Richard M. Valelly THE AMERICAN PRESIDENCY Charles O. Jones THE AMERICAN REVOLUTION Robert J. Allison AMERICAN SLAVERY Heather Andrea Williams THE AMERICAN WEST Stephen Aron AMERICAN WOMEN’S HISTORY Susan Ware ANAESTHESIA Aidan O’Donnell ANALYTIC PHILOSOPHY Michael Beaney ANARCHISM Colin Ward ANCIENT ASSYRIA Karen Radner ANCIENT EGYPT Ian Shaw ANCIENT EGYPTIAN ART AND ARCHITECTURE Christina Riggs ANCIENT GREECE Paul Cartledge THE ANCIENT NEAR EAST Amanda H. -
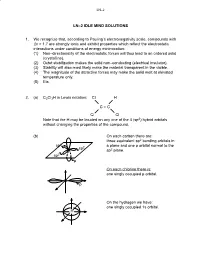
LN–2 IDLE MIND SOLUTIONS 1. We Recognize That, According To
LN–2 LN–2 IDLE MIND SOLUTIONS 1. We recognize that, according to Pauling’s electronegativity scale, compounds with Δx > 1.7 are strongly ionic and exhibit properties which reflect the electrostatic interactions under conditions of energy minimization: (1) Non–directionality of the electrostatic forces will thus lead to an ordered solid (crystalline). (2) Octet stabilization makes the solid non–conducting (electrical insulator). (3) Stability will also most likely make the material transparent in the visible. (4) The magnitude of the attractive forces may make the solid melt at elevated temperature only. (5) Etc. 2. (a) C2Cl3H in Lewis notation: Cl H C = C Cl Cl Note that the H may be located on any one of the 4 (sp2) hybrid orbitals without changing the properties of the compound. (b) On each carbon there are: three equivalent sp2 bonding orbitals in sp2 a plane and one p orbital normal to the sp2 sp2 plane. sp2 p On each chlorine there is: one singly occupied p orbital. Cl p On the hydrogen we have: one singly occupied 1s orbital. H LN–2 σ 2. (c) 2 sp2–sp2 orbitals → 1σ C–C π 2 p–p orbitals → 1π C–C 6 bonds 3 sp2–p orbitals → 3σ C–Cl 1 sp2–s orbital → 1σ C–H 3. Silicon (Si) is a group 4 element like C (carbon) and wil also exhibit sp3 hybridization: 4 sp3 orbitals at B.A. = 109o. Unlike carbon, silicon cannot form double or triple bonds (by lateral p orbital overlap) because the atom is too large to permit lateral overlap! (a) Si + 2Cl2 → SiCl4 Si is in the center of a tetrahedron with the chlorine atoms as the four corners. -

And Tipt-Based High-Temperature Shape Memory Alloys: a Review on Recent Advances
metals Review TiPd- and TiPt-Based High-Temperature Shape Memory Alloys: A Review on Recent Advances Yoko Yamabe-Mitarai 1,2 1 Department of Advanced Materials Science, Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa-shi, Chiba 277-8561, Japan; [email protected] 2 National Institute for Materials Science, 1-2-1 Sengen, Tsukuba, Ibaraki 305-0047, Japan Received: 30 September 2020; Accepted: 12 November 2020; Published: 18 November 2020 Abstract: In this paper high-temperature shape memory alloys based on TiPd and TiPt are reviewed. The effect of the alloying elements in ternary TiPd and TiPt alloys on phase transformation and strain recovery is also discussed. Generally, the addition of alloying elements decreases the martensitic transformation temperature and improves the strength of the martensite and austenite phases. Additionally, it also decreases irrecoverable strain, but without perfect recovery due to plastic deformation. With the aim to improve the strength of high-temperature shape memory alloys, multi-component alloys, including medium- and high-entropy alloys, have been investigated and proposed as new structural materials. Notably, it was discovered that the martensitic transformation temperature could be controlled through a combination of the constituent elements and alloys with high austenite finish temperatures above 500 ◦C. The irrecoverable strain decreased in the multi-component alloys compared with the ternary alloys. The repeated thermal cyclic test was effective toward obtaining perfect strain recoveries in multi-component alloys, which could be good candidates for high-temperature shape memory alloys. Keywords: high-temperature shape memory alloys; titanium palladium; titanium platinum; multi-component alloys; medium-entropy alloys; high-entropy alloys 1. -

Week 1 Slides
Adventures in Chemistry Instructor: Abi Oyeyemi Week 1 States of matter Boiling point, Melting point Atoms Types of Chemistry Hazards PPE Atoms consist of: Neutrons Protons Electrons 0 + - In In shells of atoms Nucleus States of Matter Solid, liquid, gas Week 2 Flame tests Orbitals Periodic table Octet Groups and Periods Experiment: Making a non-Newtonian Fluid Periodic Table Groups Each group tells how many electrons are in the outer shell. In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements. The elements in each group have the same number of electrons in the outer orbital. Those outer electrons are also called valence electrons Periodic table 118 elements total 8 groups Group 1- Alkali metals Group 2- Alkali earth metals Group 7 –Halogens Group 8-Noble gases Elements structure Includes abbreviated name of element Atomic number-number of protons Always same number of protons and neutrons Mass number- average no. of neutrons and protons Amu: atomic mass units Isotopes Periodic table: Periods A period in the periodic table is a row of chemical elements. All elements in a row have the same number of electron shells/orbitals Week 3 More on the periodic table Electronic configuration Isotopes Chemical vs Physical changes Compounds vs Mixtures Ionic and Covalent bonds Demonstration Lava Lamp Activity: Making models Why do elements react? Octet, want a full outer shell How? By taking or giving electrons (ionic bonding) Or by sharing electrons (covalent bonding) Compounds- A compound is a substance formed when two or more chemical elements are chemically bonded together. -

Zerohack Zer0pwn Youranonnews Yevgeniy Anikin Yes Men
Zerohack Zer0Pwn YourAnonNews Yevgeniy Anikin Yes Men YamaTough Xtreme x-Leader xenu xen0nymous www.oem.com.mx www.nytimes.com/pages/world/asia/index.html www.informador.com.mx www.futuregov.asia www.cronica.com.mx www.asiapacificsecuritymagazine.com Worm Wolfy Withdrawal* WillyFoReal Wikileaks IRC 88.80.16.13/9999 IRC Channel WikiLeaks WiiSpellWhy whitekidney Wells Fargo weed WallRoad w0rmware Vulnerability Vladislav Khorokhorin Visa Inc. Virus Virgin Islands "Viewpointe Archive Services, LLC" Versability Verizon Venezuela Vegas Vatican City USB US Trust US Bankcorp Uruguay Uran0n unusedcrayon United Kingdom UnicormCr3w unfittoprint unelected.org UndisclosedAnon Ukraine UGNazi ua_musti_1905 U.S. Bankcorp TYLER Turkey trosec113 Trojan Horse Trojan Trivette TriCk Tribalzer0 Transnistria transaction Traitor traffic court Tradecraft Trade Secrets "Total System Services, Inc." Topiary Top Secret Tom Stracener TibitXimer Thumb Drive Thomson Reuters TheWikiBoat thepeoplescause the_infecti0n The Unknowns The UnderTaker The Syrian electronic army The Jokerhack Thailand ThaCosmo th3j35t3r testeux1 TEST Telecomix TehWongZ Teddy Bigglesworth TeaMp0isoN TeamHav0k Team Ghost Shell Team Digi7al tdl4 taxes TARP tango down Tampa Tammy Shapiro Taiwan Tabu T0x1c t0wN T.A.R.P. Syrian Electronic Army syndiv Symantec Corporation Switzerland Swingers Club SWIFT Sweden Swan SwaggSec Swagg Security "SunGard Data Systems, Inc." Stuxnet Stringer Streamroller Stole* Sterlok SteelAnne st0rm SQLi Spyware Spying Spydevilz Spy Camera Sposed Spook Spoofing Splendide -

Durham E-Theses
Durham E-Theses A spectroscopic study of some halogeno-complexes of tellurium (IV) Gorrell, Ian Barnes How to cite: Gorrell, Ian Barnes (1983) A spectroscopic study of some halogeno-complexes of tellurium (IV), Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/7890/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk A SPECTROSCOPIC STUDY OF SOME HALOGENO- COMPLEXES OF TELLURIUM (IV) by Ian Barnes Gorrell A thesis submitted in part fulfilment of the requirements for the degree of Master of Science in the University of Durham. The copyright of this thesis rests with the author. April 19 8 3 No quotation from it should be published without his prior written consent and information derived from it should be acknowledged. -5. OFC. 1983 TO MY MOTHER and MY FATHER1 S MEMORY' iii ACKNOWLEDGMENTS I wish to express my gratitude towards the late Professor T.C.