Chemical Synthesis and Steady State Characterization of a Nanocrystalline Lithium Cobalt Oxide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Improving the Stability of High-Voltage Lithium Cobalt Oxide with a Multifunctional Electrolyte Additive: Interfacial Analyses
nanomaterials Article Improving the Stability of High-Voltage Lithium Cobalt Oxide with a Multifunctional Electrolyte Additive: Interfacial Analyses Xing-Qun Liao 1,2, Feng Li 2, Chang-Ming Zhang 2, Zhou-Lan Yin 1,*, Guo-Cong Liu 3,* and Jin-Gang Yu 1,3,* 1 College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China; [email protected] 2 Research Institute of Highpower International, Huizhou 516057, China; fl[email protected] (F.L.); [email protected] (C.-M.Z.) 3 School of Chemistry and Materials Engineering, Huizhou University, Huizhou 516007, China * Correspondence: [email protected] (Z.-L.Y.); [email protected] (G.-C.L.); [email protected] (J.-G.Y.); Tel./Fax: +86-731-88879616 (Z.-L.Y.); +86-731-88879616 (J.-G.Y.) Abstract: In recent years, various attempts have been made to meet the increasing demand for high energy density of lithium-ion batteries (LIBs). The increase in voltage can improve the capacity and the voltage platform performance of the electrode materials. However, as the charging voltage increases, the stabilization of the interface between the cathode material and the electrolyte will decrease, causing side reactions on both sides during the charge–discharge cycling, which seriously affects the high-temperature storage and the cycle performance of LIBs. In this study, a sulfate additive, dihydro-1,3,2-dioxathiolo[1,3,2]dioxathiole 2,2,5,5-tetraoxide (DDDT), was used as an effi- cient multifunctional electrolyte additive for high-voltage lithium cobalt oxide (LiCoO2). Nanoscale Citation: Liao, X.-Q.; Li, F.; Zhang, protective layers were formed on the surfaces of both the cathode and the anode electrodes by C.-M.; Yin, Z.-L.; Liu, G.-C.; Yu, J.-G. -

Session 1 Sources and Availability of Materials for Lithium Batteries
Session 1: Sources and Availability of Materials for Lithium Batteries Session 1 Sources and Availability of Materials for Lithium Batteries Adrian Griffin Managing Director, Lithium Australia NL ABSTRACT Lithium, as a feedstock for the battery industry, originates from two primary sources: hard-rock (generally spodumene and petalite), and brines. Brine processing results in the direct production of lithium chemicals, whereas the output from hard-rock production is tradeable mineral concentrates that require downstream processing prior to delivery, as refined chemicals, into the battery market. The processors of the concentrates, the 'converters', are the major constraint in a supply chain blessed with abundant mineral feed. The battery industry must overcome the constraints imposed by the converters, and this can be achieved through the application of the Sileach™ process, which produces lithium chemicals from concentrates direct, without the need for roasting. The cathode chemistries of the most efficient lithium batteries have a common thread – a high dependence on cobalt. Battery manufacturers consume around 40% of the current production of cobalt, a by-product of the nickel and copper industries. This means cobalt is at a tipping point – production will not keep up with demand. In the short term, the solution lies in developing alternative cathode compositions, while in the longer term recycling may be the answer. Lithium Australia NL is researching the application of its Sileach™ process to waste batteries to achieve a high-grade, low-cost source of battery materials and, in so doing, ease the supply constraints on cathode metals. To ensure that the battery industry is sustainable, better utilisation of mineral resources, more efficient processing technology, an active battery reprocessing capacity and less reliance on cobalt as a cathode material are all necessary. -

Lithium Data Sheet
98 LITHIUM (Data in metric tons of lithium content unless otherwise noted) Domestic Production and Use: The only lithium production in the United States was from a brine operation in Nevada. Two companies produced a wide range of downstream lithium compounds in the United States from domestic or imported lithium carbonate, lithium chloride, and lithium hydroxide. Domestic production data were withheld to avoid disclosing company proprietary data. Although lithium markets vary by location, global end-use markets are estimated as follows: batteries, 65%; ceramics and glass, 18%; lubricating greases, 5%; polymer production, 3%; continuous casting mold flux powders, 3%; air treatment, 1%; and other uses, 5%. Lithium consumption for batteries has increased significantly in recent years because rechargeable lithium batteries are used extensively in the growing market for portable electronic devices and increasingly are used in electric tools, electric vehicles, and grid storage applications. Lithium minerals were used directly as ore concentrates in ceramics and glass applications. Salient Statistics—United States: 2015 2016 2017 2018 2019e Production W W W W W Imports for consumption 2,750 3,140 3,330 3,420 2,500 Exports 1,790 1,520 1,960 1,660 1,700 Consumption, estimated1 2,000 3,000 3,000 3,000 2,000 Price, annual average, battery-grade lithium carbonate, dollars per metric ton2 6,500 8,650 15,000 17,000 13,000 Employment, mine and mill, number 70 70 70 70 70 Net import reliance3 as a percentage of estimated consumption >25 >50 >50 >50 >25 Recycling: One domestic company has recycled lithium metal and lithium-ion batteries since 1992 at its facility in British Columbia, Canada. -

Lithium 2017
2017 Minerals Yearbook LITHIUM [ADVANCE RELEASE] U.S. Department of the Interior September 2020 U.S. Geological Survey Lithium By Brian W. Jaskula Domestic survey data and tables were prepared by Annie Hwang, statistical assistant. In the United States, one lithium brine operation with an cobalt oxide and 2,160 kg of lithium-nickel-cobalt-aluminum associated lithium carbonate plant operated in Silver Peak, oxide (Defense Logistics Agency Strategic Materials, 2017). At NV. Domestic and imported lithium carbonate, lithium yearend 2017, the NDS held 540 kg of lithium-cobalt oxide and chloride, and lithium hydroxide were consumed directly 1,620 kg of lithium-nickel-cobalt-aluminum oxide. in industrial applications and used as raw materials for downstream lithium compounds. In 2017, lithium consumption Production in the United States was estimated to be equivalent to The U.S. Geological Survey (USGS) collected domestic 3,000 metric tons (t) of elemental lithium (table 1) [16,000 t production data for lithium from a voluntary canvass of the of lithium carbonate equivalent (LCE)], primarily owing to only U.S. lithium carbonate producer, Rockwood Lithium Inc. demand for lithium-based battery, ceramic and glass, grease, (a subsidiary of Albemarle Corp. of Charlotte, NC). Production pharmaceutical, and polymer products. In 2017, the gross weight and stock data collected from Rockwood Lithium were withheld of lithium compounds imported into the United States increased from publication to avoid disclosing company proprietary data. by 7% and the gross weight of exports increased by 29% from The company’s 6,000-metric-ton-per-year (t/yr) Silver Peak those in 2016. -

(Li-Ion) Batteries
Safety Data Sheet Validity Period: 02-Oct-2021 to Version 3 Issue Date: 09-Oct-2013 02-Oct-2022 1. IDENTIFICATION Product Identifier Product Name Lithium-Ion Battery Other means of identification SDS # GLI-006 Synonyms Lithiated Cobalt Oxide, Li-Ion Secondary Battery, Li-Ion Rechargeable Battery. Recommended use of the chemical and restrictions on use Recommended Use Battery. Details of the supplier of the safety data sheet GlobTek, Inc. Distributor Veterans Drive , NJ 07647 : 201-784-1000 Emergency Telephone Number INFOTRAC 1-352-323-3500 (International) Emergency Telephone (24 hr) 1-800-535-5053 (North America) 2. HAZARDS IDENTIFICATION Emergency Overview Safety Data Sheets (SDS) are a sub-requirement of the Occupational Safety and Health Administration (OSHA) Hazard Communication Standard, 29 CFR Subpart 1910.1200. This Hazard Communication Standard does not apply to various subcategories including anything defined by OSHA as an "article". OSHA has defined "article" as a manufactured item other than a fluid or particle; (i) which is formed to a specific shape or design during manufacture; (ii) which has end use function(s) dependent in whole or in part upon its shape or design during end use; and (iii) which under normal conditions of use does not release more than very small quantities, e.g. minute or trace amounts of a hazardous chemical, and does not pose a physical hazard or health risk to employees. Because all of our batteries are defined as "articles", they are exempt from the requirements of the Hazard Communication Standard, hence an SDS is not required. However, this Safety Data Sheet (SDS) contains valuable information critical to the safe handling and proper use of this product. -

Electron Transport and Ion Diffusivity Through the Solid
ELECTRON TRANSPORT AND ION DIFFUSIVITY THROUGH THE SOLID ELECTROLYTE INTERPHASE IN LITHIUM ION BATTERIES WITH SILICON ANODES A Dissertation by LAURA ELENA BENITEZ Submitted to the Office of Graduate and Professional Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Chair of Committee, Jorge M. Seminario Committee Members, Jun Zou Perla B. Balbuena Jose Silva-Martinez Head of Department, Miroslav M. Begovic August 2017 Major Subject: Electrical Engineering Copyright 2017 Laura Elena Benitez ABSTRACT Lithium-ion batteries (LIB) are the best option among batteries for portable electronic, power tools, and electric vehicles due to their higher energy storage, higher power, and lighter weight than other battery technologies such as Ni-based or lead acid. However, Li-ion batteries still face challenges such as safety, life, performance, and cost. One way to contribute to the solutions of these challenges and, consequently, improve the performance of Li-ion cells is to develop and design more stable passivation films at the electrode-electrolyte interface. Therefore, having a better understanding of the molecular processes that lead to the nucleation, growth, structure and morphology, as well as the electron and ion transport properties of the solid electrolyte interphase (SEI) is highly important for the development of new or improved lithium-ion batteries. In this work, computational methods, which allow studying phenomena not easily observable with experimental techniques, are used to study the electron transfer characteristics and the lithium ion diffusivity of the materials found in the SEI film formed in LIB with silicon anodes. First, ab initio computational methods are used to study the electron transfer through selected finite models of SEI films formed at the anode-electrolyte interface. -
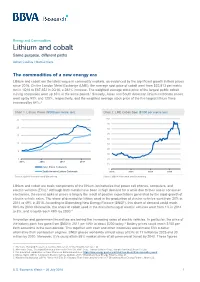
Lithium and Cobalt Same Purpose, Different Paths
Energy and Commodities Lithium and cobalt Same purpose, different paths Adrian Casillas / Marcial Nava The commodities of a new energy era Lithium and cobalt are the latest vogue in commodity markets, as evidenced by the significant growth in their prices since 2016. On the London Metal Exchange (LME), the average spot price of cobalt went from $22,813 per metric ton in 1Q16 to $87,352 in 2Q18, a 282% increase. The weighted average stock price of the largest public cobalt mining companies went up 85% in the same period.1 Similarly, Asian and South American lithium-carbonate prices went up by 93% and 120%, respectively, and the weighted average stock price of the five largest lithium firms increased by 84%.2 Chart 1. Lithium Prices ($’000 per metric ton) Chart 2. LME Cobalt Spot ($’000 per metric ton) 25 100 90 20 80 70 15 60 10 50 40 5 30 0 20 2015 2016 2017 2018 10 Asia Lithium Carbonate 0 South America Lithium Carbonate 2015 2016 2017 2018 Source: BBVA Research and Bloomberg Source: BBVA Research and Bloomberg Lithium and cobalt are basic components of the lithium-ion batteries that power cell phones, computers, and electric vehicles (EVs).3 Although both metals have been in high demand for a while due to their use in consumer electronics, the recent spike in prices is largely the result of positive expectations generated by the rapid growth of electric vehicle sales. The share of demand for lithium used in the production of electric vehicles went from 20% in 2014 to 49% in 2018. -

New Advances in Lithium Ion Battery Fuel Gauging Final
New Advances in Lithium Ion Battery Monitoring Jörn Tinnemeyer Vice President - Research & Development Cadex Electronics Inc. 22000 Fraserwood Way, Richmond BC, Canada V6W 1J6 [email protected] or www.cadex.com Abstract: In the last decade, lithium ion batteries have Importantly, though, the praises of Li-ion batteries must dominated the market place: first being used in portable be tempered by several key disadvantages in using this consumer products, and now in more industrial and chemistry. Namely, they safely operate within a very transport-based applications. One necessary requirement limited range of conditions; that is, the manufacturers of of lithium ion batteries -- irrespective of the particular Li-ion battery packs must be vigilant when developing the application of interest -- is to gauge how much energy the protection circuitry and a safe milieu for the battery battery contains and how long a given application can (McDowall et al., 2007, Van Schalkwijk et al., 2002). run before the battery needs to be recharged. The precise monitoring and management of lithium ion batteries has The precise monitoring and management of Li-ion proven to be difficult to achieve, especially as the battery batteries also presents a noteworthy problem -- this starts to age. Here, I describe a novel patented approach problem being the focus of this article. Irrespective of the that Cadex Electronics Inc., is developing, which assesses application being considered, the end-user needs to know the state of charge and state of health of lithium ion when the battery is fully charged and when the battery batteries by directly measuring the concentration of lithium will run out of power. -

Think Corner Research Note Battery Materials Value Chains
Think Corner Research Note Battery Materials Value Chains Demand, Capacity and Challenges Michelle Michot Foss, Ph.D. Gürcan Gülen, Ph.D. Rahul Verma Chief Energy Economist, Senior Energy Economist, Graduate Research Assistant Program Manager Research Scientist Chen-Hao Tsai Daniel Quijano Brent Elliott, Ph.D. Senior Energy Economist, Energy Economist, Research Research Associate, BEG Research Associate Scientist Associate II Economic Minerals ABSTRACT Our research premise is that demand for battery materials would hinge on battery chemistry driven by applications, within a context of extractive industry dynamics. Our analysis indicates that 75% utilization rates at existing production capacity coupled with upcoming projects can satisfy most of the expected demand for both lithium and cobalt in the short run. However, high-growth scenarios require new capacity and/or significantly higher rates of utilization in existing facilities. The influence of geopolitical risks, growth in other applications, and development of new applications could push demand in excess of production capacity. Government regulations in response to local interests, environmental concerns and future sustainability have influenced material supply in the past and may continue. While many factors could ease pressure for new production capacity, development of new applications like grid- energy storage and use of lithium-ion batteries in heavy vehicles have the potential to significantly increase demand beyond what is typically expected in the literature. Lithium will -

Insights Into Layered Oxide Cathodes for Rechargeable Batteries
molecules Review Insights into Layered Oxide Cathodes for Rechargeable Batteries Julia H. Yang 1, Haegyeom Kim 2 and Gerbrand Ceder 1,2,* 1 Department of Materials Science and Engineering, UC Berkeley, Berkeley, CA 94720, USA; [email protected] 2 Materials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA; [email protected] * Correspondence: [email protected] Abstract: Layered intercalation compounds are the dominant cathode materials for rechargeable Li-ion batteries. In this article we summarize in a pedagogical way our work in understanding how the structure’s topology, electronic structure, and chemistry interact to determine its electrochemical performance. We discuss how alkali–alkali interactions within the Li layer influence the voltage profile, the role of the transition metal electronic structure in dictating O3-structural stability, and the mechanism for alkali diffusion. We then briefly delve into emerging, next-generation Li-ion cathodes that move beyond layered intercalation hosts by discussing disordered rocksalt Li-excess structures, a class of materials which may be essential in circumventing impending resource limitations in our era of clean energy technology. Keywords: layered oxide cathodes; alkali–alkali interactions; electronic structure; Li diffusion Citation: Yang, J.H.; Kim, H.; Ceder, G. Insights into Layered Oxide Cathodes for Rechargeable Batteries. 1. Introduction to the O3 Structure Molecules 2021, 26, 3173. https:// Rechargeable Li-ion batteries have enabled a wireless revolution and are currently doi.org/10.3390/molecules26113173 the dominant technology used to power electric vehicles and provide resilience to a grid powered by renewables. Research in the 1970s to create superconductors by modifying Academic Editors: Stephane Jobic, the carrier density of chalcogenides through intercalation [1] transitioned into energy Claude Delmas and storage when Whittingham demonstrated in 1976 a rechargeable battery using the layered Myung-Hwan Whangbo TiS2 cathode and Li metal anode [2]. -

Science Journals
SCIENCE ADVANCES | RESEARCH ARTICLE ELECTROPLATING 2017 © The Authors, some rights reserved; Electroplating lithium transition metal oxides exclusive licensee American Association 1 2 2 1 2 2 for the Advancement Huigang Zhang, * Hailong Ning, * John Busbee, Zihan Shen, Chadd Kiggins, Yuyan Hua, of Science. Distributed 2 2 2 3 3,4 1 Janna Eaves, Jerome Davis III, Tan Shi, Yu-Tsun Shao, Jian-Min Zuo, Xuhao Hong, under a Creative 1 1 1 3 5 Yanbin Chan, Shuangbao Wang, Peng Wang, Pengcheng Sun, Sheng Xu, Commons Attribution Jinyun Liu,3 Paul V. Braun2,3,4,6,7* NonCommercial License 4.0 (CC BY-NC). Materials synthesis often provides opportunities for innovation. We demonstrate a general low-temperature (260°C) molten salt electrodeposition approach to directly electroplate the important lithium-ion (Li-ion) battery cathode materials LiCoO2, LiMn2O4, and Al-doped LiCoO2. The crystallinities and electrochemical capacities of the electroplated oxides are comparable to those of the powders synthesized at much higher temperatures (700° to 1000°C). This new growth method significantly broadens the scope of battery form factors and func- tionalities, enabling a variety of highly desirable battery properties, including high energy, high power, and unprecedented electrode flexibility. INTRODUCTION transition metals at incorrect valance states, and disorder in the crystal Lithium transition metal oxides (LTMOs), which are typically synthe- structure (21, 22). sized in powder form via solid-state reactions at 700° to 1000°C, are Here, we report a general -

A Review on the Separation of Lithium Ion from Leach Liquors of Primary and Secondary Resources by Solvent Extraction with Commercial Extractants
processes Article A Review on the Separation of Lithium Ion from Leach Liquors of Primary and Secondary Resources by Solvent Extraction with Commercial Extractants Thi Hong Nguyen 1,2 and Man Seung Lee 1,* 1 Department of Advanced Materials Science & Engineering, Institute of Rare Metal, Mokpo National University, Jeollanamdo 534-729, Korea; [email protected] 2 College of Natural Sciences, Can Tho University, Can Tho City 900000, Viet Nam * Correspondence: [email protected]; Tel.: +82-61-450-2492 Received: 10 April 2018; Accepted: 9 May 2018; Published: 12 May 2018 Abstract: The growing demand for lithium necessitates the development of an efficient process to recover it from three kinds of solutions, namely brines as well as acid and alkaline leach liquors of primary and secondary resources. Therefore, the separation of lithium(I) from these solutions by solvent extraction was reviewed in this paper. Lithium ions in brines are concentrated by removing other metal salts by crystallization with solar evaporation. In the case of ores and secondary resources, roasting followed by acid/alkaline leaching is generally employed to dissolve the lithium. Since the compositions of brines, alkaline and acid solutions are different, different commercial extractants are employed to separate and recover lithium. The selective extraction of Li(I) over other metals from brines or alkaline solutions is accomplished using acidic extractants, their mixture with neutral extractants, and neutral extractants mixed with chelating extractants in the presence of ferric chloride (FeCl3). Among these systems, tri-n-butyl phosphate (TBP)- methyl isobutyl ketone (MIBK)-FeCl3 and tri-n-octyl phosphine oxide (TOPO)- benzoyltrifluoroacetone (HBTA) are considered to be promising for the selective extraction and recovery of Li(I) from brines and alkaline solutions.