Low Back Pain and Sciatica in Over 16S: Assessment and Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

What Are the Acute Treatments for Migraine and How Are They Used?
2. Acute Treatment CQ II-2-1 What are the acute treatments for migraine and how are they used? Recommendation The mainstay of acute treatment for migraine is pharmacotherapy. The drugs used include (1) acetaminophen, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans and (5) antiemetics. Stratified treatment according to the severity of migraine is recommended: use NSAIDs such as aspirin and naproxen for mild to moderate headache, and use triptans for moderate to severe headache, or even mild to moderate headache when NSAIDs were ineffective in the past. It is necessary to give guidance and cautions to patients having acute attacks, and explain the methods of using medications (timing, dose, frequency of use) and medication use during pregnancy and breast-feeding. Grade A Background and Objective The objective of acute treatment is to resolve the migraine attack completely and rapidly and restore the patient’s normal functions. An ideal treatment should have the following characteristics: (1) resolves pain and associated symptoms rapidly; (2) is consistently effective; (3) no recurrence; (4) no need for additional use of medication; (5) no adverse effects; (6) can be administered by the patients themselves; and (7) low cost. Literature was searched to identify acute treatments that satisfy the above conditions. Comments and Evidence The acute treatment drugs for migraine generally include (1) acetaminophens, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans, and (5) antiemetics. For severe migraines including status migrainosus and migraine attacks refractory to treatment, (6) anesthetics, and (7) corticosteroids (dexamethasone) are used (Tables 1 and 2).1)-9) There are two approaches to the selection and sequencing of these medications: “step care” and “stratified care”. -

WHO Pharmaceuticals Newsletters
2021 WHO Pharmaceuticals No.1 NEWSLETTER The WHO Pharmaceuticals Newsletter provides you with the latest information on the safety of medicines WHO Vision for Medicines Safety and legal actions taken by regulatory authorities around No country left behind: the world. It also provides signals based on information worldwide pharmacovigilance for safer medicines, safer patients derived from the WHO global database of individual case safety reports, VigiBase. In addition, this edition of the Newsletter includes a The aim of the Newsletter is to disseminate regulatory summary of discussions and key recommendations of information on the safety of Advisory Committee on Safety of Medicinal Products pharmaceutical products, (ACSoMP) Seventeenth meeting. based on communications received from our network of national pharmacovigilance centres and other sources such as specialized bulletins and journals, as well as partners in WHO. The information is produced in the form of résumés in English, full texts of which may be obtained on request from: Safety and Vigilance: Medicines, EMP-HIS, World Health Organization, 1211 Geneva 27, Switzerland, Contents E -mail address: [email protected] This Newsletter is also available at: Regulatory matters http://www.who.int/medicines Safety of medicines Signal Feature WHO Pharmaceuticals Newsletter No. 1, 2021 ISBN 978-92-4-002136-5 (electronic version) ISBN 978-92-4-002137-2 (print version) © World Health Organization 2021 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. -

Torticollis Is a Clinical Diagnosis Based on Head Tilt in Association with a Rotatory Deviation of the Cranium
24. Define type of torticollis? Torticollis is a clinical diagnosis based on head tilt in association with a rotatory deviation of the cranium. Congenital muscular torticollis is the most common type of torticollis. It presents in the newborn period. Its cause is unknown, but it has been hypothesized to arise from compression of the soft tissues of the neck during delivery, resulting in a compartment syndrome. Radiographs of the cervical spine should be obtained to rule out congenital vertebral anomalies. Clinical examination reveals spasm of the sternocleidomastoid muscle on the same side as the tilt causing the typical posture of head tilt toward the tightened muscle and chin rotation to the opposite side. Initial treatment is stretching and is successful in up to 90% of patients during the first year of life. Surgery is considered for persistent deformity after 1 year of age. Common problems noted in patients with congenital muscular torticollis include congenital hip dysplasia and plagiocephaly (facial asymmetry). Source: Spine Secret Plus, 2nd ed. page 256. 25. What clinical problems result from basilar impression? Patients present with a short neck, painful cervical motion, and asymmetric of the skull and face. Additional clinical problems include nuchal pain, vertigo, long tract signs with associated cerebellar ataxia, and lower cranial nerve involvement resulting in dysarthria and dysphagia Source: Spine Secret Plus, 2nd ed. page 258 26. What is Steel’s Rule of Third? Steel noted that the area of the spinal canal at the C1 leve in a normal person could be divided into equal thirds with one third occupied by the odontoid process, one third by the spinal cord, and one third as empty space. -

Big Pain Assays Aren't a Big Pain with the Raptor Biphenyl LC Column
Featured Application: 231 Pain Management and Drugs of Abuse Compounds in under 10 Minutes by LC-MS/MS Big Pain Assays Aren’t a Big Pain with the Raptor Biphenyl LC Column • 231 compounds, 40+ isobars, 10 drug classes, 22 ESI- compounds in 10 minutes with 1 column. • A Raptor SPP LC column with time-tested Restek Biphenyl selectivity is the most versatile, multiclass-capable LC column available. • Achieve excellent separation of critical isobars with no tailing peaks. • Run fast and reliable high-throughput LC-MS/MS analyses with increased sensitivity using simple mobile phases. The use of pain management drugs is steadily increasing. As a result, hospital and reference labs are seeing an increase in patient samples that must be screened for a wide variety of pain management drugs to prevent drug abuse and to ensure patient safety and adherence to their medication regimen. Thera- peutic drug monitoring can be challenging due to the low cutoff levels, potential matrix interferences, and isobaric drug compounds. To address these chal- lenges, many drug testing facilities are turning to liquid chromatography coupled with mass spectrometry (LC-MS/MS) for its increased speed, sensitivity, and specificity. As shown in the analysis below, Restek’s Raptor Biphenyl column is ideal for developing successful LC-MS/MS pain medication screening methodologies. With its exceptionally high retention and unique selectivity, 231 multiclass drug compounds and metabolites—including over 40 isobars—can be analyzed in just 10 minutes. In addition, separate panels have been optimized on the Raptor Biphenyl column specifically for opioids, antianxiety drugs, barbiturates, NSAIDs and analgesics, antidepressants, antiepileptics, antipsychotics, hallucinogens, and stimulants for use during confirmation and quantitative analyses. -

PMBJP Product.Pdf
Sr. Drug Generic Name of the Medicine Unit Size MRP Therapeutic Category No. Code Analgesic & Antipyretic / Muscle 1 1 Aceclofenac 100mg and Paracetamol 325 mg Tablet 10's 10's 8.00 relaxants Analgesic & Antipyretic / Muscle 2 2 Aceclofenac Tablets IP 100mg 10's 10's 4.37 relaxants Acetaminophen 325 + Tramadol Hydrochloride 37.5 film Analgesic & Antipyretic / Muscle 3 4 10's 8.00 coated Tablet 10's relaxants Analgesic & Antipyretic / Muscle 4 5 ASPIRIN Tablets IP 150 mg 14's 14's 2.70 relaxants DICLOFENAC 50 mg+ PARACETAMOL 325 mg+ Analgesic & Antipyretic / Muscle 5 6 10's 11.30 CHLORZOXAZONE 500 mg Tablets 10's relaxants Diclofenac Sodium 50mg + Serratiopeptidase 10mg Tablet Analgesic & Antipyretic / Muscle 6 8 10's 12.00 10's relaxants Analgesic & Antipyretic / Muscle 7 9 Diclofenac Sodium (SR) 100 mg Tablet 10's 10's 6.12 relaxants Analgesic & Antipyretic / Muscle 8 10 Diclofenac Sodium 25mg per ml Inj. IP 3 ml 3 ml 2.00 relaxants Analgesic & Antipyretic / Muscle 9 11 Diclofenac Sodium 50 mg Tablet 10's 10's 2.90 relaxants Analgesic & Antipyretic / Muscle 10 12 Etoricoxilb Tablets IP 120mg 10's 10's 33.00 relaxants Analgesic & Antipyretic / Muscle 11 13 Etoricoxilb Tablets IP 90mg 10's 10's 25.00 relaxants Analgesic & Antipyretic / Muscle 12 14 Ibuprofen 400 mg + Paracetamol 325 mg Tablet 10's 15's 5.50 relaxants Analgesic & Antipyretic / Muscle 13 15 Ibuprofen 200 mg film coated Tablet 10's 10's 1.80 relaxants Analgesic & Antipyretic / Muscle 14 16 Ibuprofen 400 mg film coated Tablet 10's 15's 3.50 relaxants Analgesic & Antipyretic -

Sciatica and Chronic Pain
Sciatica and Chronic Pain Past, Present and Future Robert W. Baloh 123 Sciatica and Chronic Pain Robert W. Baloh Sciatica and Chronic Pain Past, Present and Future Robert W. Baloh, MD Department of Neurology University of California, Los Angeles Los Angeles, CA, USA ISBN 978-3-319-93903-2 ISBN 978-3-319-93904-9 (eBook) https://doi.org/10.1007/978-3-319-93904-9 Library of Congress Control Number: 2018952076 © Springer International Publishing AG, part of Springer Nature 2019 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors, and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, express or implied, with respect to the material contained herein or for any errors or omissions that may have been made. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. -

Chapter 25 Mechanisms of Action of Antiepileptic Drugs
Chapter 25 Mechanisms of action of antiepileptic drugs GRAEME J. SILLS Department of Molecular and Clinical Pharmacology, University of Liverpool _________________________________________________________________________ Introduction The serendipitous discovery of the anticonvulsant properties of phenobarbital in 1912 marked the foundation of the modern pharmacotherapy of epilepsy. The subsequent 70 years saw the introduction of phenytoin, ethosuximide, carbamazepine, sodium valproate and a range of benzodiazepines. Collectively, these compounds have come to be regarded as the ‘established’ antiepileptic drugs (AEDs). A concerted period of development of drugs for epilepsy throughout the 1980s and 1990s has resulted (to date) in 16 new agents being licensed as add-on treatment for difficult-to-control adult and/or paediatric epilepsy, with some becoming available as monotherapy for newly diagnosed patients. Together, these have become known as the ‘modern’ AEDs. Throughout this period of unprecedented drug development, there have also been considerable advances in our understanding of how antiepileptic agents exert their effects at the cellular level. AEDs are neither preventive nor curative and are employed solely as a means of controlling symptoms (i.e. suppression of seizures). Recurrent seizure activity is the manifestation of an intermittent and excessive hyperexcitability of the nervous system and, while the pharmacological minutiae of currently marketed AEDs remain to be completely unravelled, these agents essentially redress the balance between neuronal excitation and inhibition. Three major classes of mechanism are recognised: modulation of voltage-gated ion channels; enhancement of gamma-aminobutyric acid (GABA)-mediated inhibitory neurotransmission; and attenuation of glutamate-mediated excitatory neurotransmission. The principal pharmacological targets of currently available AEDs are highlighted in Table 1 and discussed further below. -

Eperisone-Induced Anaphylaxis: Pharmacovigilance Data and Results of Allergy Testing
Allergy Asthma Immunol Res. 2019 Jan;11(1):e18 https://doi.org/10.4168/aair.2019.11.e18 pISSN 2092-7355·eISSN 2092-7363 Original Article Eperisone-Induced Anaphylaxis: Pharmacovigilance Data and Results of Allergy Testing Kyung Hee Park,1,2 Sang Chul Lee,1,2 Ji Eun Yuk,2 Sung-Ryeol Kim,1,2 Jae-Hyun Lee,1,2 Jung-Won Park1,2* 1Division of Allergy and Immunology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea 2Institute of Allergy, Yonsei University College of Medicine, Seoul, Korea Received: Aug 6, 2018 ABSTRACT Revised: Sep 10, 2018 Accepted: Sep 22, 2018 Purpose: Eperisone is an oral muscle relaxant used in musculoskeletal disorders causing Correspondence to muscle spasm and pain. For more effective pain control, eperisone is usually prescribed Jung-Won Park, MD, PhD together with nonsteroidal anti-inflammatory drugs (NSAIDs). As such, eperisone may Division of Allergy and Immunology, have been overlooked as the cause of anaphylaxis compared with NSAIDs. This study aimed Department of Internal Medicine, Yonsei to analyze the adverse drug reaction (ADR) reported in Korea and suggest an appropriate University College of Medicine, 50-1 Yonsei-ro, diagnostic approach for eperisone-induced anaphylaxis. Seodaemun-gu, Seoul 03722, Korea. Tel: +82- 2-2228-1961; Fax: +82-2-393-6884; Methods: We reviewed eperisone-related pharmacovigilance data (Korea Institute of Drug E-mail: [email protected] Safety-Korea Adverse Event Reporting System [KIDS-KAERS]) reported in Korea from 2010 to 2015. ADRs with causal relationship were selected. Clinical manifestations, severity, Copyright © 2019 The Korean Academy of outcomes, and re-exposure information were analyzed. -
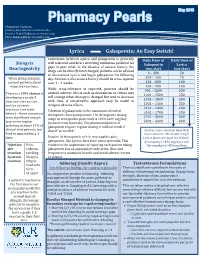
Lyrica Gabapentin: an Easy Switch!
Pharmacist Contacts: [email protected]; [email protected]; [email protected] Lyrica Gabapentin: An Easy Switch! Conversion between Lyrica and gabapentin is generally Daily Dose of Daily Dose of Shingrix well tolerated and direct switching minimizes potential for Gabapentin Lyrica Reactogenicity gaps in pain relief. In the absence of seizure history, the (mg/day) (mg/day) drugs can be directly interchanged; patients can be advised 0 – 300 50 to discontinue Lyrica and begin gabapentin the following When giving Shingrix, day. Patients with a seizure history should be cross-tapered 301 – 450 75 counsel patients about over 1 – 4 weeks. 451 – 600 100 expected reactions. 601 – 900 150 While cross-tolerance is expected, patients should be 901 – 1200 200 advised adverse effects such as drowsiness or edema may There is a 10% chance of 1201 – 1500 250 still emerge when therapy is changed but tend to decrease developing a grade 3 1501 – 1800 300 injection site reaction with time. A conservative approach may be useful to 1801 – 2100 350 and/or systemic mitigate adverse effects. reactions (see table 2101 – 2400 400 Titration of gabapentin to the maximum tolerated 2401 – 2700 450 below) – these symptoms therapeutic dose is important. The therapeutic dosing 2701 – 3000 500 were significant enough range in neuropathic pain trials is 1800-3600 mg/day to prevent regular (normal renal function). The pharmacokinetics of 3001 – 3600 600 activities in about 17% of gabapentin require regular dosing, it will not work if clinical trial patients, but dosed “as needed.” Studies show minimal benefit & tend to pass within 2-3 more adverse effects when high days. -

Efficacy and Tolerability of Eperisone in Patients with Spastic Palsy: a Cross-Over, Placebo-Controlled Dose-Ranging Trial
European Review for Medical and Pharmacological Sciences 2009; 13: 365-370 Efficacy and tolerability of eperisone in patients with spastic palsy: a cross-over, placebo-controlled dose-ranging trial N. BRESOLINA, C. ZUCCAB, A. PECORIC AInstitute of Clinical Neurology, University of Milan, I.R.C.C.S. Ospedale Maggiore Policlinico, Milan (Italy) BNeurophysiopathology Unit, IRCCS “E. Medea”, Bosisio Parini (LC) (Italy) CMedical Department Eisai Srl, San Donato Milanese, Milano (Italy) Abstract. – Background and Objectives: Introduction Central muscle relaxants are a clinical option in patients with spastic palsy. Eperisone is a cen- tral muscle relaxant used in several conditions, Spastic palsy is a clinical condition character- but its therapeutic potential in spastic palsy ized by a velocity-dependent increase of muscle needs to be verified. This dose-ranging trial tone due to “parapyramidal” disturbance of the compares two doses of eperisone in patients inhibitory afferents to the second motor neuron1. with spastic palsy associated to cerebral or spinal diseases. This condition may represent a major impedi- Patients and Methods: In this randomized, ment to a full functional recovery and social re- placebo-controlled, double-blind, three-way habilitation in patients with lesions of the pyra- cross-over study, patients (18-75 years) with midal system, which could follow different dis- spastic palsy received eperisone 150 mg/day, eases, as stroke or multiple sclerosis. eperisone 300 mg/day, or placebo for 8 weeks. Central muscle relaxants, such as baclofen, ti- Treatment periods lasted for 14 days. Objective zanidine, diazepam and dantrolene, represent an clinical parameters (intensity of spasticity and physiological reflexes) and functional parame- interesting clinical option in patients with spastic ters (walking capability, capability to climb palsy, since they reduce spasticity and improve stairs, rigidity) were measured. -

Can Eyes Cause Neck Pain? International Journal of Therapy and Rehabilitation, 23 (10)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Northumbria Research Link Citation: Hood, Wendy and Hood, Martin (2016) Can eyes cause neck pain? International Journal of Therapy and Rehabilitation, 23 (10). pp. 499-504. ISSN 1741-1645 Published by: Mark Allen Publishing URL: http://dx.doi.org/10.12968/ijtr.2016.23.10.499 <http://dx.doi.org/10.12968/ijtr.2016.23.10.499> This version was downloaded from Northumbria Research Link: http://nrl.northumbria.ac.uk/28627/ Northumbria University has developed Northumbria Research Link (NRL) to enable users to access the University’s research output. Copyright © and moral rights for items on NRL are retained by the individual author(s) and/or other copyright owners. Single copies of full items can be reproduced, displayed or performed, and given to third parties in any format or medium for personal research or study, educational, or not-for-profit purposes without prior permission or charge, provided the authors, title and full bibliographic details are given, as well as a hyperlink and/or URL to the original metadata page. The content must not be changed in any way. Full items must not be sold commercially in any format or medium without formal permission of the copyright holder. The full policy is available online: http://nrl.northumbria.ac.uk/policies.html This document may differ from the final, published version of the research and has been made available online in accordance with publisher policies. To read and/or cite from the published version of the research, please visit the publisher’s website (a subscription may be required.) Sensory Integration: Can eyes cause neck pain? Abstract This article is an analysis of literature relating to ocular-motor imbalance and the potential postural consequences.