Functional Morphology and Diversity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anchialine Cave Biology in the Era of Speleogenomics Jorge L
International Journal of Speleology 45 (2) 149-170 Tampa, FL (USA) May 2016 Available online at scholarcommons.usf.edu/ijs International Journal of Speleology Off icial Journal of Union Internationale de Spéléologie Life in the Underworld: Anchialine cave biology in the era of speleogenomics Jorge L. Pérez-Moreno1*, Thomas M. Iliffe2, and Heather D. Bracken-Grissom1 1Department of Biological Sciences, Florida International University, Biscayne Bay Campus, North Miami FL 33181, USA 2Department of Marine Biology, Texas A&M University at Galveston, Galveston, TX 77553, USA Abstract: Anchialine caves contain haline bodies of water with underground connections to the ocean and limited exposure to open air. Despite being found on islands and peninsular coastlines around the world, the isolation of anchialine systems has facilitated the evolution of high levels of endemism among their inhabitants. The unique characteristics of anchialine caves and of their predominantly crustacean biodiversity nominate them as particularly interesting study subjects for evolutionary biology. However, there is presently a distinct scarcity of modern molecular methods being employed in the study of anchialine cave ecosystems. The use of current and emerging molecular techniques, e.g., next-generation sequencing (NGS), bestows an exceptional opportunity to answer a variety of long-standing questions pertaining to the realms of speciation, biogeography, population genetics, and evolution, as well as the emergence of extraordinary morphological and physiological adaptations to these unique environments. The integration of NGS methodologies with traditional taxonomic and ecological methods will help elucidate the unique characteristics and evolutionary history of anchialine cave fauna, and thus the significance of their conservation in face of current and future anthropogenic threats. -

Ostracoda an Introduction.Pdf
The Ostracoda (from Wikipedia, 5/5/2009: http://en.wikipedia.org/wiki/Ostracod) Ostracoda is a class of the Crustacea, sometimes known as the seed shrimp because of their appearance. Ostracods are small crustaceans, typically around one mm in size, but varying between 0.2 to 30 mm, laterally compressed and protected by a bivalve-like, chitinous or calcareous valve or "shell". The hinge of the two valves is in the upper, dorsal region of the body. Some 65,000 species (13,000 of which are extant taxa) have been identified, grouped into several orders. This group may not be monophyletic. Ostracod taxa are grouped into a Class based on gross morphology. Ecologically, marine ostracods can be part of the zooplankton or (most commonly) they are part of the benthos, living on or inside the upper layer of the sea floor. Many ostracods, especially the Podocopida, are also found in fresh water and some are known from humid continental forest soils. The body consists of a cephalon (head), separated from the thorax by a slight constriction. The segmentation is unclear. The abdomen is regressed or absent whereas the adult gonads are relatively large. There are 5–8 pairs of appendages. The branchial plates are responsible for oxygenation. The epidermal cells may also secrete calcium carbonate after the chitinous layer is formed, resulting in a chalk layer enveloped by chitin. This calcification is not equally pronounced in all orders. During every instar transition, the old carapace (chitinous and calcified) is rejected and a new, larger is formed and calcified. The outer lamella calcifies completely, while the inner lamella calcifies partially, with the rest remaining chitinous. -

Zootaxa,Crustacean Classification
Zootaxa 1668: 313–325 (2007) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2007 · Magnolia Press ISSN 1175-5334 (online edition) Crustacean classification: on-going controversies and unresolved problems* GEOFF A. BOXSHALL Department of Zoology, The Natural History Museum, Cromwell Road, London SW7 5BD, United Kingdom E-mail: [email protected] *In: Zhang, Z.-Q. & Shear, W.A. (Eds) (2007) Linnaeus Tercentenary: Progress in Invertebrate Taxonomy. Zootaxa, 1668, 1–766. Table of contents Abstract . 313 Introduction . 313 Treatment of parasitic Crustacea . 315 Affinities of the Remipedia . 316 Validity of the Entomostraca . 318 Exopodites and epipodites . 319 Using of larval characters in estimating phylogenetic relationships . 320 Fossils and the crustacean stem lineage . 321 Acknowledgements . 322 References . 322 Abstract The journey from Linnaeus’s original treatment to modern crustacean systematics is briefly characterised. Progress in our understanding of phylogenetic relationships within the Crustacea is linked to continuing discoveries of new taxa, to advances in theory and to improvements in methodology. Six themes are discussed that serve to illustrate some of the major on-going controversies and unresolved problems in the field as well as to illustrate changes that have taken place since the time of Linnaeus. These themes are: 1. the treatment of parasitic Crustacea, 2. the affinities of the Remipedia, 3. the validity of the Entomostraca, 4. exopodites and epipodites, 5. using larval characters in estimating phylogenetic rela- tionships, and 6. fossils and the crustacean stem-lineage. It is concluded that the development of the stem lineage concept for the Crustacea has been dominated by consideration of taxa known only from larval or immature stages. -

Guzik IS07040.Qxd
CSIRO PUBLISHING www.publish.csiro.au/journals/is Invertebrate Systematics, 2008, 22, 205–216 Phylogeography of the ancient Parabathynellidae (Crustacea:Bathynellacea) from the Yilgarn region of Western Australia M. T. Guzik A,E, K. M. Abrams A, S. J. B. Cooper A,B, W. F. HumphreysC, J.-L. ChoD and A. D. Austin A AAustralian Centre for Evolutionary Biology and Biodiversity, School of Earth and Environmental Sciences, The University of Adelaide, SA 5005, Australia. BEvolutionary Biology Unit, South Australian Museum, North Terrace, Adelaide, SA 5000, Australia. CWestern Australian Museum, Locked Bag 49, Welshpool DC, WA 6986, Australia. DInternational Drinking Water Center, San 6-2, Yeonchuck-Dong, Daedok-Gu, Taejeon 306-711, Korea. ECorresponding author. Email: [email protected] Abstract. The crustacean order Bathynellacea is a primitive group of subterranean aquatic (stygobitic) invertebrates that typically inhabits freshwater interstitial spaces in alluvia. A striking diversity of species from the bathynellacean family Parabathynellidae have been found in the calcretes of the Yilgarn palaeodrainage system in Western Australia. Taxonomic studies show that most species are restricted in their distribution to a single calcrete, which is consistent with the findings of other phylogeographic studies of stygofauna. In this, the first molecular phylogenetic and phylogeographic study of interspecific relationships among parabathynellids, we aimed to explore the hypothesis that species are short-range endemics and restricted to single calcretes, and to investigate whether there were previously unidentified cryptic species. Analyses of sequence data based on a region of the mitochondrial (mt) DNA cytochrome c oxidase 1 gene showed the existence of divergent mtDNA lineages and species restricted in their distribution to a single calcrete, in support of the broader hypothesis that these calcretes are equivalent to closed island habitats comprising endemic taxa. -

Advanced Morphometric Techniques Applied to The
UNIVERSIDAD POLITÉCNICA DE MADRID ESCUELA TÉCNICA SUPERIOR DE INGENIEROS DE TELECOMUNICACIÓN ADVANCED MORPHOMETRIC TECHNIQUES APPLIED TO THE STUDY OF HUMAN BRAIN ANATOMY TESIS DOCTORAL Yasser Alemán Gómez Ingeniero en Tecnologías Nucleares y Energéticas Máster en Neurociencias Madrid, 2015 DEPARTAMENTO DE INGENIERÍA ELECTRÓNICA ESCUELA TÉCNICA SUPERIOR DE INGENIEROS DE TELECOMUNICACIÓN PHD THESIS ADVANCED MORPHOMETRIC TECHNIQUES APPLIED TO THE STUDY OF HUMAN BRAIN ANATOMY AUTHOR Yasser Alemán Gómez Ing. en Tecnologías Nucleares y Energéticas MSc en Neurociencias ADVISOR Manuel Desco Menéndez, MScE, MD, PhD Madrid, 2015 Departamento de Ingeniería Electrónica Escuela Técnica Superior de Ingenieros de Telecomunicación Universidad Politécnica de Madrid Ph.D. Thesis Advanced morphometric techniques applied to the study of human brain anatomy Tesis doctoral Técnicas avanzadas de morfometría aplicadas al estudio de la anatomía cerebral humana Author: Yasser Alemán Gómez Advisor: Manuel Desco Menéndez Committee: Andrés Santos Lleó Universidad Politécnica de Madrid, Madrid, Spain Javier Pascau Gonzalez-Garzón Universidad Carlos III de Madrid, Madrid, Spain Raymond Salvador Civil FIDMAG – Germanes Hospitalàries, Barcelona, Spain Pablo Campo Martínez-Lage Universidad Autónoma de Madrid, Madrid, Spain Juan Domingo Gispert López Universidad Pompeu Fabra, Barcelona, Spain María Jesús Ledesma Carbayo Universidad Politécnica de Madrid, Madrid, Spain Juan José Vaquero López Universidad Carlos III de Madrid, Madrid, Spain Esta Tesis ha sido desarrollada en el Laboratorio de Imagen Médica de la Unidad de Medicina y Cirugía Experimental del Instituto de Investigación Sanitaria Gregorio Marañón y en colaboración con el Servicio de Psiquiatría del Niño y del Adolescente del Departamento de Psiquiatría del Hospital General Universitario Gregorio Marañón de Madrid, España. Tribunal nombrado por el Sr. -
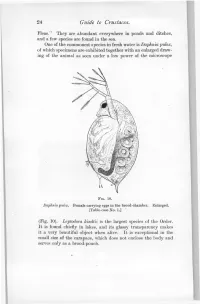
24 Guide to Crustacea
24 Guide to Crustacea. Fleas." They are abundant everywhere in ponds and ditches, and a few species are found in the sea. One of the commonest species in fresh water is Daphnia pulex, of which specimens are exhibited together with an enlarged draw- ing of the animal as seen under a low power of the microscope FIG. 10. Daphnia pulex. Female carrying eggs in the brood-chamber. Enlarged. [Table-case No. 1.] (Fig. 10). Leptodora kindtii is the largest species of the Order. It is found chiefly in lakes, and its glassy transparency makes it a very beautiful object when alive. It is exceptional in the small size of the carapace, which does not enclose the body and serves only as a brood-pouch. Ostracoda. 25 Sub-class II.—OSTRACODA. (Table-ease No. 1.) The number of somites, as indicated by the appendages, is smaller than in any other Crustacea, there being, at most, only two pairs of trunk-limbs behind those belonging to the head- region. The carapace forms a bivalved shell completely en- closing the body and limbs. There is a large, and often leg-like, palp on the mandible. The antennules and antennae are used for creeping or swimming. The Ostracoda (Fig. 10) are for the most part extremely minute animals, and only one or two of the larger species can be exhibited. They occur abundantly in fresh water and in FIG. 11. Shells of Ostracoda, seen from the side. A. Philomedes brenda (Myodocopa) ; B. Cypris fuscata (Podocopa); ('. Cythereis ornata (Podocopa): all much enlarged, n., Notch characteristic of the Myodocopa; e., the median eye ; a., mark of attachment of the muscle connecting the two valves of the shell. -

15 International Symposium on Ostracoda
Berliner paläobiologische Abhandlungen 1-160 6 Berlin 2005 15th International Symposium on Ostracoda In Memory of Friedrich-Franz Helmdach (1935-1994) Freie Universität Berlin September 12-15, 2005 Abstract Volume (edited by Rolf Kohring and Benjamin Sames) 2 ---------------------------------------------------------------------------------------------------------------------------------------- Preface The 15th International Symposium on Ostracoda takes place in Berlin in September 2005, hosted by the Institute of Geological Sciences of the Freie Universität Berlin. This is the second time that the International Symposium on Ostracoda has been held in Germany, following the 5th International Symposium in Hamburg in 1974. The relative importance of Ostracodology - the science that studies Ostracoda - in Germany is further highlighted by well-known names such as G.W. Müller, Klie, Triebel and Helmdach, and others who stand for the long tradition of research on Ostracoda in Germany. During our symposium in Berlin more than 150 participants from 36 countries will meet to discuss all aspects of living and fossil Ostracoda. We hope that the scientific communities working on the biology and palaeontology of Ostracoda will benefit from interesting talks and inspiring discussions - in accordance with the symposium's theme: Ostracodology - linking bio- and geosciences We wish every participant a successful symposium and a pleasant stay in Berlin Berlin, July 27th 2005 Michael Schudack and Steffen Mischke CONTENT Schudack, M. and Mischke, S.: Preface -

First Insights Into the Subterranean Crustacean Bathynellacea
RESEARCH ARTICLE First Insights into the Subterranean Crustacean Bathynellacea Transcriptome: Transcriptionally Reduced Opsin Repertoire and Evidence of Conserved Homeostasis Regulatory Mechanisms Bo-Mi Kim1, Seunghyun Kang1, Do-Hwan Ahn1, Jin-Hyoung Kim1, Inhye Ahn1,2, Chi- Woo Lee3, Joo-Lae Cho4, Gi-Sik Min3*, Hyun Park1,2* a1111111111 1 Unit of Polar Genomics, Korea Polar Research Institute, Incheon, South Korea, 2 Polar Sciences, a1111111111 University of Science & Technology, Yuseong-gu, Daejeon, South Korea, 3 Department of Biological a1111111111 Sciences, Inha University, Incheon, South Korea, 4 Nakdonggang National Institute of Biological Resources, a1111111111 Sangju, South Korea a1111111111 * [email protected] (HP); [email protected] (GM) Abstract OPEN ACCESS Bathynellacea (Crustacea, Syncarida, Parabathynellidae) are subterranean aquatic crusta- Citation: Kim B-M, Kang S, Ahn D-H, Kim J-H, Ahn I, Lee C-W, et al. (2017) First Insights into the ceans that typically inhabit freshwater interstitial spaces (e.g., groundwater) and are occa- Subterranean Crustacean Bathynellacea sionally found in caves and even hot springs. In this study, we sequenced the whole Transcriptome: Transcriptionally Reduced Opsin transcriptome of Allobathynella bangokensis using RNA-seq. De novo sequence assembly Repertoire and Evidence of Conserved produced 74,866 contigs including 28,934 BLAST hits. Overall, the gene sequences were Homeostasis Regulatory Mechanisms. PLoS ONE 12(1): e0170424. doi:10.1371/journal. most similar to those of the waterflea Daphnia pulex. In the A. bangokensis transcriptome, pone.0170424 no opsin or related sequences were identified, and no contig aligned to the crustacean visual Editor: Peng Xu, Xiamen University, CHINA opsins and non-visual opsins (i.e. -

Crustaceans Topics in Biodiversity
Topics in Biodiversity The Encyclopedia of Life is an unprecedented effort to gather scientific knowledge about all life on earth- multimedia, information, facts, and more. Learn more at eol.org. Crustaceans Authors: Simone Nunes Brandão, Zoologisches Museum Hamburg Jen Hammock, National Museum of Natural History, Smithsonian Institution Frank Ferrari, National Museum of Natural History, Smithsonian Institution Photo credit: Blue Crab (Callinectes sapidus) by Jeremy Thorpe, Flickr: EOL Images. CC BY-NC-SA Defining the crustacean The Latin root, crustaceus, "having a crust or shell," really doesn’t entirely narrow it down to crustaceans. They belong to the phylum Arthropoda, as do insects, arachnids, and many other groups; all arthropods have hard exoskeletons or shells, segmented bodies, and jointed limbs. Crustaceans are usually distinguishable from the other arthropods in several important ways, chiefly: Biramous appendages. Most crustaceans have appendages or limbs that are split into two, usually segmented, branches. Both branches originate on the same proximal segment. Larvae. Early in development, most crustaceans go through a series of larval stages, the first being the nauplius larva, in which only a few limbs are present, near the front on the body; crustaceans add their more posterior limbs as they grow and develop further. The nauplius larva is unique to Crustacea. Eyes. The early larval stages of crustaceans have a single, simple, median eye composed of three similar, closely opposed parts. This larval eye, or “naupliar eye,” often disappears later in development, but on some crustaceans (e.g., the branchiopod Triops) it is retained even after the adult compound eyes have developed. In all copepod crustaceans, this larval eye is retained throughout their development as the 1 only eye, although the three similar parts may separate and each become associated with their own cuticular lens. -

A Practical Introduction to Landmark-Based Geometric Morphometrics
A PRACTICAL INTRODUCTION TO LANDMARK-BASED GEOMETRIC MORPHOMETRICS MARK WEBSTER Department of the Geophysical Sciences, University of Chicago, 5734 South Ellis Avenue, Chicago, IL 60637 and H. DAVID SHEETS Department of Physics, Canisius College, 2001 Main Street, Buffalo, NY 14208 ABSTRACT.—Landmark-based geometric morphometrics is a powerful approach to quantifying biological shape, shape variation, and covariation of shape with other biotic or abiotic variables or factors. The resulting graphical representations of shape differences are visually appealing and intuitive. This paper serves as an introduction to common exploratory and confirmatory techniques in landmark-based geometric morphometrics. The issues most frequently faced by (paleo)biologists conducting studies of comparative morphology are covered. Acquisition of landmark and semilandmark data is discussed. There are several methods for superimposing landmark configu- rations, differing in how and in the degree to which among-configuration differences in location, scale, and size are removed. Partial Procrustes superimposition is the most widely used superimposition method and forms the basis for many subsequent operations in geometric morphometrics. Shape variation among superimposed con- figurations can be visualized as a scatter plot of landmark coordinates, as vectors of landmark displacement, as a thin-plate spline deformation grid, or through a principal components analysis of landmark coordinates or warp scores. The amount of difference in shape between two configurations can be quantified as the partial Procrustes distance; and shape variation within a sample can be quantified as the average partial Procrustes distance from the sample mean. Statistical testing of difference in mean shape between samples using warp scores as variables can be achieved through a standard Hotelling’s T2 test, MANOVA, or canonical variates analysis (CVA). -

Lack of Taxonomic Differentiation in An
ARTICLE IN PRESS Molecular Phylogenetics and Evolution xxx (2005) xxx–xxx www.elsevier.com/locate/ympev Lack of taxonomic diVerentiation in an apparently widespread freshwater isopod morphotype (Phreatoicidea: Mesamphisopidae: Mesamphisopus) from South Africa Gavin Gouws a,¤, Barbara A. Stewart b, Conrad A. Matthee a a Evolutionary Genomics Group, Department of Botany and Zoology, University of Stellenbosch, Private Bag X1, Matieland 7602, South Africa b Centre of Excellence in Natural Resource Management, University of Western Australia, 444 Albany Highway, Albany, WA 6330, Australia Received 20 December 2004; revised 2 June 2005; accepted 2 June 2005 Abstract The unambiguous identiWcation of phreatoicidean isopods occurring in the mountainous southwestern region of South Africa is problematic, as the most recent key is based on morphological characters showing continuous variation among two species: Mesam- phisopus abbreviatus and M. depressus. This study uses variation at 12 allozyme loci, phylogenetic analyses of 600 bp of a COI (cyto- chrome c oxidase subunit I) mtDNA fragment and morphometric comparisons to determine whether 15 populations are conspeciWc, and, if not, to elucidate their evolutionary relationships. Molecular evidence suggested that the most easterly population, collected from the Tsitsikamma Forest, was representative of a yet undescribed species. Patterns of diVerentiation and evolutionary relation- ships among the remaining populations were unrelated to geographic proximity or drainage system. Patterns of isolation by distance were also absent. An apparent disparity among the extent of genetic diVerentiation was also revealed by the two molecular marker sets. Mitochondrial sequence divergences among individuals were comparable to currently recognized intraspeciWc divergences. Sur- prisingly, nuclear markers revealed more extensive diVerentiation, more characteristic of interspeciWc divergences. -

Visual Adaptations in Crustaceans: Chromatic, Developmental, and Temporal Aspects
FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: ©2003 Springer‐Verlag. This manuscript is an author version with the final publication available at http://www.springerlink.com and may be cited as: Marshall, N. J., Cronin, T. W., & Frank, T. M. (2003). Visual Adaptations in Crustaceans: Chromatic, Developmental, and Temporal Aspects. In S. P. Collin & N. J. Marshall (Eds.), Sensory Processing in Aquatic Environments. (pp. 343‐372). Berlin: Springer‐Verlag. doi: 10.1007/978‐0‐387‐22628‐6_18 18 Visual Adaptations in Crustaceans: Chromatic, Developmental, and Temporal Aspects N. Justin Marshall, Thomas W. Cronin, and Tamara M. Frank Abstract Crustaceans possess a huge variety of body plans and inhabit most regions of Earth, specializing in the aquatic realm. Their diversity of form and living space has resulted in equally diverse eye designs. This chapter reviews the latest state of knowledge in crustacean vision concentrating on three areas: spectral sensitivities, ontogenetic development of spectral sen sitivity, and the temporal properties of photoreceptors from different environments. Visual ecology is a binding element of the chapter and within this framework the astonishing variety of stomatopod (mantis shrimp) spectral sensitivities and the environmental pressures molding them are examined in some detail. The quantity and spectral content of light changes dra matically with depth and water type and, as might be expected, many adaptations in crustacean photoreceptor design are related to this governing environmental factor. Spectral and temporal tuning may be more influenced by bioluminescence in the deep ocean, and the spectral quality of light at dawn and dusk is probably a critical feature in the visual worlds of many shallow-water crustaceans.