Final Assessment Report Proposal P295
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Legislative Assembly Hansard 1971
Queensland Parliamentary Debates [Hansard] Legislative Assembly THURSDAY, 2 SEPTEMBER 1971 Electronic reproduction of original hardcopy Matters of Public Interest [2 SEPTEMBER 1971] Q1iestions Upon Notice 337 THURSDAY, 2 SEPTEMBER 1971 Mr. SPEAKER (Hon. D. E. Nicholson, Murrumba) read prayers and took the chair at 11 a.m. PAPERS The following papers were laid on the table:- Order in Council under the Medical Act 1939-1969 Regulations under- Ambulance Services Act 1967-1970. The Hospitals Acts, 1936 to 1967. QUESTIONS UPON NOTICE MEAT EXPORTS AND IMPORTS Mr. Houston, pursuant to notice, asked The Minister for Primary Industries,- What quantities of (a) beef, (b) veal, (c) mutton, (d) lamb and (e) pigmeats, were (i) exported from and (ii) imported into Queensland during 1970-71? Answer:- "Information from the Commonwealth Statistician indicates the following quanti ties exported overseas from Queensland during 1970-71 :-Beef-fresh, chilled and frozen, 354,708,831 lb.; Veal-fresh, chilled and frozen, 6,633,080 lb.; Mutton -fresh, chilled and frozen, 19,434,521 338 Questions Upon Notice (2 SEPTEMBER 1971] Questions Upon Notice lb.; Lamb-fresh, chilled and frozen. King George Square, urging that a hoard 589,997 lb.; Pigrneats-fresh, chilled and ing, extending the length of the project, frozen, 822,036 lb. These figures are be erected to ensure the continued safety subject to revision. Imports from overseas of the public using the Square as the sources are negligible. Information on project progresses and will the Brisbane interstate imports and exports is not yet City Council, which has been notified on available." numerous occasions of this situation, not permit the erection of such a hoarding? SHORT-WEIGIIT BREAD (2) What is the actual situation and Mr. -

20021 Food Number and Item GI 2 Serve GL 3 Glucose S
REVISED INTERNATIONAL TABLE OF GLYCEMIC INDEX (GI) AND GLYCEMIC LOAD (GL)—20021 Food Number and Item GI 2 Serve GL 3 glucose size per =100 grams serve BAKERY PRODUCTS Cakes 1 Angel food cake (Loblaw's, Toronto, Canada) 67 50 19 2 Banana cake, made with sugar 47±8 80 18 3 Banana cake, made without sugar 55±10 80 16 Chocolate cake made from packet mix with chocolate frosting (Betty Crocker, General 4 38±3 111 20 Mills Inc., Minneapolis, MN, USA) Cupcake, strawberry-iced (Squiggles, Farmland, Grocery Holdings, Tooronga, Vic, 5 73±12 38 19 Australia) Lamingtons (sponge dipped in chocolate and coconut) (Farmland, Grocery Holdings, 6 87±17 50 25 Australia) 7 Pound cake (Sara Lee Canada, Bramalea, Canada) 54 53 15 8 Sponge cake, plain 46±6 63 17 9 Vanilla cake made from packet mix with vanilla frosting (Betty Crocker, USA) 42±4 111 24 10 Croissant (Food City, Toronto, Canada) 67 57 17 11 Crumpet (Dempster's Corporate Foods Ltd., Etobicoke, Canada) 69 50 13 12 Doughnut, cake type (Loblaw's, Canada) 76 47 17 13 Flan cake (Weston's Bakery, Toronto, Canada) 65 70 31 14 Muffins Apple, made with sugar4 44±6 60 13 Apple, made without sugar4 48±10 60 9 Apple, oat, sultana, made from packet mix (Defiance Milling Co., Acacia Ridge, Qld, 54±4 50 14 Australia) Apricot, coconut and honey, made from packet mix (Defiance Milling Co., Australia) 60±4 50 16 Banana, oat and honey, made from packet mix (Defiance Milling Co., Australia) 65±11 50 17 Bran (Culinar Inc., Grandma Martin's Muffins, Aurora, Canada) 60 57 15 Blueberry (Culinar Inc., Canada) 59 57 17 Carrot (Culinar Inc., Canada) 62 57 20 Chocolate butterscotch, made from packet mix (Defiance Milling Co., Australia) 53±5 50 15 Corn muffin, low-amylose5 102 57 30 Corn muffin, high-amylose5 49 Oatmeal, muffin, made from mix (Quaker Oats Co. -

Annual Report 2008–09
Annual Report 2008–09 reducing the incidence and impact of cancer in the act for over 40 years The Australian Capital Territory (ACT) Working in the Australian Capital Territory to reduce the incidence and impact of cancer Cancer Council ACT Cancer Council ACT (the Council) is a non PROGRAMS AND SERVICES government, not-for-profit community organisation that aims to promote a Cancer Information Service healthier community by reducing the >> information on all aspects of cancer incidence and impact of cancer in the ACT >> Cancer Council Helpline 13 11 20 region. The Council depends largely on free publications and lending library the generosity of the ACT and surrounding >> community providing donations and Cancer Prevention and Early Detection Program supporting fundraising initiatives. >> promotes cancer prevention behaviours and participation in appropriate early detection programs Memberships >> Smoking Cessation and Prevention Service – Quitline 13 7848 Cancer Council ACT, together with other member organisations in each state and – quit smoking courses and seminars territory, is a member of Cancer Council – The ACT Vulnerable Youth: Reducing Chronic Disease Risk Factors Project Australia. Through this membership >> SunSmart services the Council is a member of the Asian – National SunSmart Schools and SunSmart Early Childhood Program and Pacific Federation of Organisations for Cancer Research and Control; the – SunSmart workplace information sessions International Non-Governmental Coalition – Cancer Council ACT Shop Against Tobacco; and the International >> health promotion booths and information displays Union For Health Promotion and Education. Supportive Care Service Cancer Council ACT is also a member of >> facilitated support groups for people affected by cancer the International Union against Cancer (UICC). -

Annual Report and Accounts 2019 Accounts and Report Annual
Associated British Foods plc Annual Report and Accounts 2019 ANNUAL REPORT AND ACCOUNTS 2019 Grocery Contents Strategic report 1 Financial headlines 2 Our businesses at a glance Read more 4 Chairman’s statement page 12 6 Chief Executive’s statement 8 Group business model and strategy 10 Key performance indicators 12 Operating review 12 Grocery Sugar 22 Sugar 30 Agriculture 34 Ingredients 40 Retail 50 Financial review 53 Responsibility 62 Principal risks and uncertainties 67 Viability statement Governance Read more 68 Board of directors page 22 70 Corporate governance 83 Remuneration report 107 Directors’ report 110 Statement of directors’ responsibilities Agriculture 111 I ndependent auditor’s report Financial statements 119 Consolidated income statement 120 Consolidated statement of comprehensive income 121 Consolidated balance sheet 122 Consolidated cash flow statement 123 Consolidated statement of changes in equity 124 Significant accounting policies Read more 131 Accounting estimates page 30 and judgements 132 Notes forming part of the financial statements 176 Company financial statements Ingredients 183 Progress report 184 Company directory Read more page 34 Retail Read more page 00 Read more page 40 Well-loved ABOUT household brands ASSOCIATED 9/10 BRITISH UK households use FOODS our brands Our purpose is to provide safe, nutritious, affordable food and clothing that is great value for money. A leader in our markets Grocery Retail Our grocery brands occupy leading Primark is the largest clothing, positions in markets across the footwear and accessories globe. In the UK, nine out of ten retailer in the UK, and also has a households use our brands. significant store portfolio in ten European countries and in the US. -
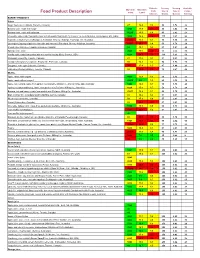
Glycemic Index
Diabetic Serving Serving Available Glycemic Glycemic Carb Size in Size in Carbs / Food Product Description Index Load Choices Grams Ounces Serving BAKERY PRODUCTS Cakes Angel food cake (Loblaw's, Toronto, Canada) 67 19.4 1.9 50 1.76 29 Banana cake, made with sugar 47±8 17.9 2.5 80 2.82 38 Banana cake, made without sugar 55±10 16.0 1.9 80 2.82 29 Chocolate cake made from packet mix with chocolate frosting (Betty Crocker, General Mills Inc., Minneapolis, MN, USA) 38±3 19.8 3.5 111 3.91 52 Cupcake, strawberry-iced (Squiggles, Farmland, Grocery Holdings, Tooronga, Vic, Australia) 73±12 19.0 1.7 38 1.34 26 Lamingtons (sponge dipped in chocolate and coconut) (Farmland, Grocery Holdings, Australia) 87±17 25.2 1.9 50 1.76 29 Pound cake (Sara Lee Canada, Bramalea, Canada) 54 15.1 1.9 53 1.87 28 Sponge cake, plain 46±6 16.6 2.4 63 2.22 36 Vanilla cake made from packet mix with vanilla frosting (Betty Crocker, USA) 42±4 24.2 3.9 111 3.91 58 Croissant (Food City, Toronto, Canada) 67 17.5 1.7 57 2.01 26 Crumpet (Dempster's Corporate Foods Ltd., Etobicoke, Canada) 69 13.1 1.3 50 1.76 19 Doughnut, cake type (Loblaw's, Canada) 76 17.4 1.5 47 1.66 23 Flan cake (Weston's Bakery, Toronto, Canada) 65 31.2 3.2 70 2.47 48 Muffins Apple, made with sugar4 44±6 12.8 1.9 60 2.12 29 Apple, made without sugar4 48±10 9.0 1.3 60 2.12 19 Apple, oat, sultana, made from packet mix (Defiance Milling Co., Acacia Ridge, Qld, Australia) 54±4 14.0 1.7 50 1.76 26 Apricot, coconut and honey, made from packet mix (Defiance Milling Co., Australia) 60±4 15.6 1.7 50 1.76 26 Banana, oat and honey, made from packet mix (Defiance Milling Co., Australia) 65±11 16.9 1.7 50 1.76 26 Bran (Culinar Inc., Grandma Martin's Muffins, Aurora, Canada) 60 14.6 1.6 57 2.01 24 Blueberry (Culinar Inc., Canada) 59 17.3 1.9 57 2.01 29 Carrot (Culinar Inc., Canada) 62 20.1 2.1 57 2.01 32 Chocolate butterscotch, made from packet mix (Defiance Milling Co., Australia) 53±5 15.0 1.9 50 1.76 28 Corn muffin, low-amylose5 102 29.6 1.9 57 2.01 29 Corn muffin, high-amylose5 49 Oatmeal, muffin, made from mix (Quaker Oats Co. -
2020 Responsibility Update
Responsibility Update 2020 Overview People Society and supply chains Environment Assurance statement 2 WELCOME to our Responsibility Update 2020 Our purpose has helped us get through a year like no other. At Associated British Foods, our purpose is to provide safe, nutritious, affordable food and clothing that offer great value for money. Contents 2020 has been an unprecedented year, and the personal cost associated with the COVID-19 outbreak 9 has been terrible. Our employees, customers, suppliers Overview2 Investing in our people Respecting41 the environment and communities were all put under enormous 3 A message from our Chairman 10 Health and safety 42 Climate change mitigation pressure by the pandemic and although some and Chief Executive 16 Diversity and inclusion and adaptation uncertainty remains, our group has proved to be strong 4 Making a positive contribution 19 Employee engagement 47 Natural resources and the and resilient. 5 Our breadth is one of our and development circular economy We’re incredibly proud of the flexibility, commitment greatest strengths 53 Biodiversity and healthy ecosystems and determination of our people, from our food 6 Staying true to what’s important businesses significantly increasing production to meet 7 Our approach: doing the right thing 57 Animal health and welfare unprecedented demand to our Retail division, where 8 Supporting global goals Supporting23 society and all Primark stores were forced to close. However, strengthening our supply chains together, we went above and beyond the call of duty 24 Human rights and labour rights Assurance58 statement to provide for our customers and mitigate the impacts 33 Healthy and safe products on our supply chains. -

Corporate Responsibility Report 2016 CONTENTS
Corporate Responsibility ReportCorporate 2016 every day every Doing good Doing Associated British Foods plc Corporate Responsibility Report 2016 CONTENTS OVERVIEW 1 Chief executive’s introduction 2 Measuring our social impact 4 Our businesses at a glance 6 The essence of Associated British Foods “Our purpose 8 Our approach to corporate responsibility 12 Our group priorities 13 Addressing modern slavery is to 16 Protecting our environment provide 18 Climate change 20 Water management 21 Waste management 22 Keeping our people safe 24 Promoting gender diversity 25 Supporting the community: nutri the Garfield Weston Foundation safe, tious, GROCERY 26 Introduction 28 Our environment affordable 31 Our people 34 Our supply chain food 38 Our neighbours 40 Our customers and that is SUGAR clothing 42 Introduction 44 Our environment FOR MONEY. 48 Our people GREAT VALUE 50 Our supply chain 54 Our neighbours 55 Our customers In doing these things well AGRICULTURE 56 Introduction 58 Our environment we know we contribute 60 Our people 62 Our supply chain to making MILLIONS of 64 Our neighbours 66 Our customers INGREDIENTS people’s lives better.” 68 Introduction 70 Our environment 72 Our people 75 Our neighbours 76 Our customers George Weston RETAIL Chief Executive, Associated British Foods 78 Introduction 80 Our environment 82 Our people 84 Our supply chain 88 Our neighbours ASSURANCE 90 EY’s assurance report Associated British Foods plc Corporate Responsibility Report 2016 Overview Chief executive’s introduction 1 CHIEF EXECUTIVE’S INTRODUCTION Associated British Foods is a company which thinks long term, invests consistently in its assets and finances itself conservatively. -
Download 2007-08 Report
The Cancer Council ACT Annual Report 2007–0832nd Reducing the impact of cancer in the ACT for 40 years The Australian Capital Territory (ACT) Working in the Australian Capital Territory to reduce the incidence and impact of cancer The Cancer Council ACT The Cancer Council ACT (The Council) is a non PROGRAMS AND SERVICES government not-for-profit community organisation that aims to promote a healthier community by reducing the Cancer Information Service incidence and impact of cancer in the ACT region. The Council depends largely on the generosity of the ACT > information on all aspects of cancer and surrounding community providing donations and > Cancer Council Helpline 13 11 20 supporting fundraising initiatives. > free publications and lending library Vision Cancer Prevention and Early Detection Program To promote a healthier community by reducing the > promotes cancer prevention behaviours and participation in incidence and impact of cancer in the Canberra appropriate early detection programs area through information, education, supportive care > Smoking Cessation and Prevention Service and research. – Quitline 13 7848 Values – quit smoking courses and seminars > accepting the principles of the Ottawa Charter – The ACT Vulnerable Youth Reducing Chronic Disease Risk for Health Promotion Factors Project > provision of quality programs and services > SunSmart services > working within an evidence-based paradigm – National SunSmart Schools and SunSmart Early Childhood Program > working within a community/environmental/ ecological approach -

Annual Report and Accounts 2016 Worldreginfo - 454Bddc2-114C-4Ea3-Ad78-B27b6e198a4b FINANCIAL HEADLINES
Annual Report and Accounts 2016 WorldReginfo - 454bddc2-114c-4ea3-ad78-b27b6e198a4b FINANCIAL HEADLINES Group revenue Adjusted profit before tax** Dividends per share Operating profit £13.4bn £1,071m 36.75p £1,103m Actual: +5% Constant currency: +4% Up 5% Up 5% Up 18% Adjusted operating profit* Adjusted earnings Gross investment Profit before tax £1,118m per share** £1bn £1,042m Actual: +3% Constant currency: +3% 106.2p Up 47% Up 5% Net debt Basic earnings per share £315m 103.4p Up 55% Associated British Foods is a diversified international food, ingredients and retail group with sales of £13.4bn, 130,000 employees and operations in 50 countries across Europe, southern Africa, the Americas, Asia and Australia. REVIEW OF THE YEAR ONLINE: STRATEGIC REPORT GOVERNANCE www.abf.co.uk/ar2016 IFC Financial headlines 54 Board of directors 1 Our year in review 56 Corporate governance 2 Our businesses at a glance 69 Remuneration report 4 Chairman’s statement 88 Directors’ report 6 Chief Executive’s statement 91 Statement of directors’ responsibilities 8 Group business model and strategy 92 Independent auditor’s report 10 Business strategies 12 Operating review FINANCIAL STATEMENTS 12 Grocery 20 Sugar 98 Consolidated income statement 26 Agriculture 99 Consolidated statement of 32 Ingredients comprehensive income 36 Retail 100 Consolidated balance sheet 42 Financial review 101 Consolidated cash flow statement 44 Corporate responsibility 102 Consolidated statement of changes 48 Principal risks and uncertainties in equity 103 Significant accounting policies 108 Accounting estimates and judgements 109 Notes forming part of the financial statements 149 Company financial statements IBC Progress report IBC Company directory * Before amortisation of non-operating intangibles, profits less losses on disposal of non-current assets and exceptional items. -

(Vanilla) Meal 53 Source: Randall J. Gretebeck Et Al., "Glycemic Index of Popular Sp
MET-Rx (Vanilla) meal 58 Boost (Vanilla) meal 53 Source: Randall J. Gretebeck et al., "Glycemic index of popular sports drinks and energy foods," Journal of the American Dietetic Association, March 2002, pp. 415- 417. Dates: bahri dates 50 khalas dates 36 bo ma'an dates 31 Source: Campbell J. Miller et al., "Glycemic index of 3 varieties of dates," Saudi Medical Journal, May 2002, pp. 536-538. New pasta test: ProtiPasta 28 100 10 Source: Sydney University's Glycemic Index Research Service Last modified: April 6, 2005 David Mendosa 4455 Chippewa Dr Boulder, CO 80303 E-mail: [email protected] Telephone: (303) 494- 2036 24AUC measured over 5 h, but blood samples taken at hourly intervals only. 25Potato used as reference food with a GI fixed at 80. The GI of the test food was calculated by expressing the test food's glucose AUC value as a percentage of the potato's AUC value. 26White rice was used as the reference food, but glucose was also tested and had a GI of 122. The observed GI was multiplied by 100 and then divided by 122 to convert it to a GI on the glucose scale (ie, glucose = reference food with a GI of 100). 27Blood glucose measured at 30-min intervals. 28GI for sugars calculated from the glycemic response for a meal of sugar and rolled oats minus the glycemic response for the oats alone. 29Both the test food and the reference food contained 30 g carbohydrate. 30Total weight of the test food was 25 g, whereas reference food contained 25 g available carbohydrate. -

Glycemic Index and Glycemic Load of More Than 2,480 Individual Food Items
GlycemicAdvertisement Index and Glycemic Load Advanced Search Revised International Table of Glycemic Index (GI) and Glycemic Load (GL) Values— 2008 By David Mendosa Advertisement This is the definitive table for both the glycemic index and the glycemic load. I am able to reproduce it here courtesy of the author, Professor Jennie Brand-Miller of the University of Sydney. It is based on a table in different format but no more foods published December 2008 in Diabetes Care. However, only the abstract is free online there. This table includes the glycemic index and glycemic load of more than 2,480 individual food items. Not all of them, however, are available in the United States. They represent a GI true international effort of testing around the world. of 55 The glycemic index (GI) is a numerical system of measuring how much of a rise in circulating blood sugar a carbohydrate triggers–the higher the number, the greater the is blood sugar response. So a low GI food will cause a small rise, while a high GI food will low; trigger a dramatic spike. A list of carbohydrates with their glycemic values is shown GL below. A GI is 70 or more is high, a GI of 56 to 69 inclusive is medium, and a GI of 55 of or less is low. 10 The glycemic load (GL) is a relatively new way to assess the impact of carbohydrate is consumption that takes the glycemic index into account, but gives a fuller picture than http://www.mendosa.com/gilists.htm (1 of 127) [12/17/2011 8:14:06 AM] Glycemic Index and Glycemic Load does glycemic index alone. -

2020 Creative Agencies New Business League
2020 CREATIVE AGENCIES NEW BUSINESS LEAGUE Australia / Aug 2020 ESTIMATED ESTIMATED YTD RANK THIS RANK LAST OVERALL YTD No.of AGENCY RECENT WINS WIN REVENUE RECENT LOSSES MONTH MONTH REVENUE Wins (USD $ m) (USD $m) Kmart, McDonald's, Coles, Johnson 1 1 DDB 14.6 Foxtel 12.6 7 & Johnson Coles, Emerald Foods, New Zealand 2 3 TBWA 4.8 4.8 3 Natural Adore Beauty, Libra, Red Cross 3 2 Cummins & Partners Humanitarian, Sapporo, Jeep/Fiat, 5.1 Tourism Western Australia 4.6 11 Alfa Romeo, Hesta, Heritage Bank Australian liquor marketers, Ego 4 4 Channel T 4.2 4.2 8 Pharma, Sorbent 5 5 BBDO Seafood Industry, Stake, Weis 3.4 3.4 10 Open Colleges, Tourism Western 6 6 The Monkeys 2.3 2.3 4 Australia, Rebel 7 8 72andSunny Tooheys Extra Dry, Sendle 2.3 2.3 4 Ampol, Foxtel, Fight Food Waste 8 7 Saatchi & Saatchi 2.0 2.0 4 CRC Project 9 9 MullenLowe Group Citi 1.5 1.5 2 Danone Nutricia adult health, Place 10 10 Grey Group Management NSW, GSK Consumer 1.4 1.4 3 Healthcare digital and social 11 12 Ogilvy Uncle Toby's cereals 1.3 1.3 2 12 13 Wunderman Thompson Transport for NSW - Taxi's Project 1.2 1.2 10 Dan Murphy's liquor, SSangyong 13 14 ThinkerBell Motors, Business Council of 1.1 1.1 3 Australia Val Morgan Digital, Catholic 14 18 Orchard Healthcare, World Square/JLL, 1.0 1.0 5 Cover More Insurance 15 49 M&C Saatchi Domain 1.0 1.0 1 Bupa Dental, Bupa Optical and 16 15 Edge 0.9 0.9 2 Hearing 17 20 Common Ventures Tarocash, Bank Australia 0.9 0.9 2 18 16 Isobar Finance Client 0.8 0.8 6 19 17 The Wired Agency St Luke's Care digital 0.8 0.8 2 20 19 LOUD Lipton