Population Dynamics of the Indonesian Mantis Shrimp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Learning in Stomatopod Crustaceans
International Journal of Comparative Psychology, 2006, 19 , 297-317. Copyright 2006 by the International Society for Comparative Psychology Learning in Stomatopod Crustaceans Thomas W. Cronin University of Maryland Baltimore County, U.S.A. Roy L. Caldwell University of California, Berkeley, U.S.A. Justin Marshall University of Queensland, Australia The stomatopod crustaceans, or mantis shrimps, are marine predators that stalk or ambush prey and that have complex intraspecific communication behavior. Their active lifestyles, means of predation, and intricate displays all require unusual flexibility in interacting with the world around them, imply- ing a well-developed ability to learn. Stomatopods have highly evolved sensory systems, including some of the most specialized visual systems known for any animal group. Some species have been demonstrated to learn how to recognize and use novel, artificial burrows, while others are known to learn how to identify novel prey species and handle them for effective predation. Stomatopods learn the identities of individual competitors and mates, using both chemical and visual cues. Furthermore, stomatopods can be trained for psychophysical examination of their sensory abilities, including dem- onstration of color and polarization vision. These flexible and intelligent invertebrates continue to be attractive subjects for basic research on learning in animals with relatively simple nervous systems. Among the most captivating of all arthropods are the stomatopod crusta- ceans, or mantis shrimps. These marine creatures, unfamiliar to most biologists, are abundant members of shallow marine ecosystems, where they are often the dominant invertebrate predators. Their common name refers to their method of capturing prey using a folded, anterior raptorial appendage that looks superficially like the foreleg of a praying mantis. -

Chapter 2 Political Development and Demographic Features
Cover Page The handle http://hdl.handle.net/1887/36062 holds various files of this Leiden University dissertation Author: Xiaodong Xu Title: Genesis of a growth triangle in Southeast Asia : a study of economic connections between Singapore, Johor and the Riau Islands, 1870s – 1970s Issue Date: 2015-11-04 Chapter 2 Political Development and Demographic Features A unique feature distinguishing this region from other places in the world is the dynamic socio-political relationship between different ethnic groups rooted in colonial times. Since then, both conflict and compromise have occurred among the Europeans, Malays and Chinese, as well as other regional minorities, resulting in two regional dichotomies: (1) socially, the indigenous (Malays) vs. the outsiders (Europeans, Chinese, etc.); (2) politically, the rulers (Europeans and Malay nobles) vs. those ruled (Malays, Chinese). These features have a direct impact on economic development. A retrospective survey of regional political development and demographic features are therefore needed to provide a context for the later analysis of economic development. 1. Political development The formation of Singapore, Johor and the Riau Islands was far from a sudden event, but a long process starting with the decline of the Johor-Riau Sultanate in the late eighteenth century. In order to reveal the coherency of regional political transformations, the point of departure of this political survey begins much earlier than the researched period here. Political Development and Demographic Features 23 The beginning of Western penetration (pre-1824) Apart from their geographical proximity, Singapore, Johor and the Riau Islands had also formed a natural and inseparable part of various early unified kingdoms in Southeast Asia. -

The Mediterranean Decapod and Stomatopod Crustacea in A
ANNALES DU MUSEUM D'HISTOIRE NATURELLE DE NICE Tome V, 1977, pp. 37-88. THE MEDITERRANEAN DECAPOD AND STOMATOPOD CRUSTACEA IN A. RISSO'S PUBLISHED WORKS AND MANUSCRIPTS by L. B. HOLTHUIS Rijksmuseum van Natuurlijke Historie, Leiden, Netherlands CONTENTS Risso's 1841 and 1844 guides, which contain a simple unannotated list of Crustacea found near Nice. 1. Introduction 37 Most of Risso's descriptions are quite satisfactory 2. The importance and quality of Risso's carcino- and several species were figured by him. This caused logical work 38 that most of his names were immediately accepted by 3. List of Decapod and Stomatopod species in Risso's his contemporaries and a great number of them is dealt publications and manuscripts 40 with in handbooks like H. Milne Edwards (1834-1840) Penaeidea 40 "Histoire naturelle des Crustaces", and Heller's (1863) Stenopodidea 46 "Die Crustaceen des siidlichen Europa". This made that Caridea 46 Risso's names at present are widely accepted, and that Macrura Reptantia 55 his works are fundamental for a study of Mediterranean Anomura 58 Brachyura 62 Decapods. Stomatopoda 76 Although most of Risso's descriptions are readily 4. New genera proposed by Risso (published and recognizable, there is a number that have caused later unpublished) 76 authors much difficulty. In these cases the descriptions 5. List of Risso's manuscripts dealing with Decapod were not sufficiently complete or partly erroneous, and Stomatopod Crustacea 77 the names given by Risso were either interpreted in 6. Literature 7S different ways and so caused confusion, or were entirely ignored. It is a very fortunate circumstance that many of 1. -

Pt Inti Indosawit Subur – Tungkal Ulu Group and Scheme Smallholders
PUBLIC SUMMARY REPORT RSPO SECOND ANNUAL SURVEILLANCE ASSESSMENT (ASA2) PT INTI INDOSAWIT SUBUR – TUNGKAL ULU GROUP AND SCHEME SMALLHOLDERS Jambi Province, Sumatra, INDONESIA Report Author: Haeruddin Tahir – September 2014 BSI Group Singapore Pte Ltd (Co. Reg. 1995 02096‐N) PT. BSI Group Indonesia 1 Robinson Road Menara Bidakara 2, 17th Floor Unit 5 AIA Tower #15‐01 Jl. Jend. Gatot Subroto Kav. 71‐73 Singapore 048542 Komplek Bidakara, Pancoran Tel +65 6270 0777 Jakarta Selatan 12870 ‐ Indonesia Fax +65 6270 2777 Tel +62 21 8379 3174 ‐ 77 www.bsigroup.sg Fax +62 21 8379 3287 Aryo Gustomo: [email protected] TABLE of CONTENTS page № SUMMARY......................................................................................................................................................... 1 Abbreviations Used........................................................................................................................................... 1 1.0 SCOPE OF CERTIFICATION ASSESSMENT.............................................................................................. 1 1.1 National Interpretation Used...................................................................................................................... 1 1.2 Certification Scope...................................................................................................................................... 1 1.3 Location and Maps.................................................................................................................................... -
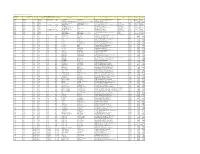
Colgate Palmolive List of Mills As of June 2018 (H1 2018) Direct
Colgate Palmolive List of Mills as of June 2018 (H1 2018) Direct Supplier Second Refiner First Refinery/Aggregator Information Load Port/ Refinery/Aggregator Address Province/ Direct Supplier Supplier Parent Company Refinery/Aggregator Name Mill Company Name Mill Name Country Latitude Longitude Location Location State AgroAmerica Agrocaribe Guatemala Agrocaribe S.A Extractora La Francia Guatemala Extractora Agroaceite Extractora Agroaceite Finca Pensilvania Aldea Los Encuentros, Coatepeque Quetzaltenango. Coatepeque Guatemala 14°33'19.1"N 92°00'20.3"W AgroAmerica Agrocaribe Guatemala Agrocaribe S.A Extractora del Atlantico Guatemala Extractora del Atlantico Extractora del Atlantico km276.5, carretera al Atlantico,Aldea Champona, Morales, izabal Izabal Guatemala 15°35'29.70"N 88°32'40.70"O AgroAmerica Agrocaribe Guatemala Agrocaribe S.A Extractora La Francia Guatemala Extractora La Francia Extractora La Francia km. 243, carretera al Atlantico,Aldea Buena Vista, Morales, izabal Izabal Guatemala 15°28'48.42"N 88°48'6.45" O Oleofinos Oleofinos Mexico Pasternak - - ASOCIACION AGROINDUSTRIAL DE PALMICULTORES DE SABA C.V.Asociacion (ASAPALSA) Agroindustrial de Palmicutores de Saba (ASAPALSA) ALDEA DE ORICA, SABA, COLON Colon HONDURAS 15.54505 -86.180154 Oleofinos Oleofinos Mexico Pasternak - - Cooperativa Agroindustrial de Productores de Palma AceiteraCoopeagropal R.L. (Coopeagropal El Robel R.L.) EL ROBLE, LAUREL, CORREDORES, PUNTARENAS, COSTA RICA Puntarenas Costa Rica 8.4358333 -82.94469444 Oleofinos Oleofinos Mexico Pasternak - - CORPORACIÓN -

Visual Adaptations in Crustaceans: Chromatic, Developmental, and Temporal Aspects
FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: ©2003 Springer‐Verlag. This manuscript is an author version with the final publication available at http://www.springerlink.com and may be cited as: Marshall, N. J., Cronin, T. W., & Frank, T. M. (2003). Visual Adaptations in Crustaceans: Chromatic, Developmental, and Temporal Aspects. In S. P. Collin & N. J. Marshall (Eds.), Sensory Processing in Aquatic Environments. (pp. 343‐372). Berlin: Springer‐Verlag. doi: 10.1007/978‐0‐387‐22628‐6_18 18 Visual Adaptations in Crustaceans: Chromatic, Developmental, and Temporal Aspects N. Justin Marshall, Thomas W. Cronin, and Tamara M. Frank Abstract Crustaceans possess a huge variety of body plans and inhabit most regions of Earth, specializing in the aquatic realm. Their diversity of form and living space has resulted in equally diverse eye designs. This chapter reviews the latest state of knowledge in crustacean vision concentrating on three areas: spectral sensitivities, ontogenetic development of spectral sen sitivity, and the temporal properties of photoreceptors from different environments. Visual ecology is a binding element of the chapter and within this framework the astonishing variety of stomatopod (mantis shrimp) spectral sensitivities and the environmental pressures molding them are examined in some detail. The quantity and spectral content of light changes dra matically with depth and water type and, as might be expected, many adaptations in crustacean photoreceptor design are related to this governing environmental factor. Spectral and temporal tuning may be more influenced by bioluminescence in the deep ocean, and the spectral quality of light at dawn and dusk is probably a critical feature in the visual worlds of many shallow-water crustaceans. -

Mill List - 2020
General Mills - Mill List - 2020 General Mills July 2020 - December 2020 Parent Mill Name Latitude Longitude RSPO Country State or Province District UML ID 3F Oil Palm Agrotech 3F Oil Palm Agrotech 17.00352 81.46973 No India Andhra Pradesh West Godavari PO1000008590 Aathi Bagawathi Manufacturing Abdi Budi Mulia 2.051269 100.252339 No Indonesia Sumatera Utara Labuhanbatu Selatan PO1000004269 Aathi Bagawathi Manufacturing Abdi Budi Mulia 2 2.11272 100.27311 No Indonesia Sumatera Utara Labuhanbatu Selatan PO1000008154 Abago Extractora Braganza 4.286556 -72.134083 No Colombia Meta Puerto Gaitán PO1000008347 Ace Oil Mill Ace Oil Mill 2.91192 102.77981 No Malaysia Pahang Rompin PO1000003712 Aceites De Palma Aceites De Palma 18.0470389 -94.91766389 No Mexico Veracruz Hueyapan de Ocampo PO1000004765 Aceites Morichal Aceites Morichal 3.92985 -73.242775 No Colombia Meta San Carlos de Guaroa PO1000003988 Aceites Sustentables De Palma Aceites Sustentables De Palma 16.360506 -90.467794 No Mexico Chiapas Ocosingo PO1000008341 Achi Jaya Plantations Johor Labis 2.251472222 103.0513056 No Malaysia Johor Segamat PO1000003713 Adimulia Agrolestari Segati -0.108983 101.386783 No Indonesia Riau Kampar PO1000004351 Adimulia Agrolestari Surya Agrolika Reksa (Sei Basau) -0.136967 101.3908 No Indonesia Riau Kuantan Singingi PO1000004358 Adimulia Agrolestari Surya Agrolika Reksa (Singingi) -0.205611 101.318944 No Indonesia Riau Kuantan Singingi PO1000007629 ADIMULIA AGROLESTARI SEI TESO 0.11065 101.38678 NO INDONESIA Adimulia Palmo Lestari Adimulia Palmo Lestari -

Length-Weight Relationships of Squilla Mantis (Linnaeus, 1758)
International Journal of Fisheries and Aquatic Studies 2018; 6(6): 241-246 E-ISSN: 2347-5129 P-ISSN: 2394-0506 (ICV-Poland) Impact Value: 5.62 Length-weight relationships of Squilla mantis (GIF) Impact Factor: 0.549 IJFAS 2018; 6(6): 241-246 (Linnaeus, 1758) (Crustacea, Stomatopoda, Squillidae) © 2018 IJFAS www.fisheriesjournal.com from Thermaikos Gulf, North-West Aegean Sea, Received: 02-09-2018 Accepted: 03-10-2018 Greece Thodoros E Kampouris (1) Marine Sciences Department, Thodoros E Kampouris, Emmanouil Kouroupakis, Marianna Lazaridou, School of the Environment, and Ioannis E Batjakas University of the Aegean, Mytilene, Lesvos Island, Greece (2) Astrolabe Marine Research, Abstract Mytilene, Lesvos Island, Greece The length-weight relationships (L-W) and the allometric growth profile of the stomatopod Squilla mantis was studied from Thermaikos Gulf, Aegean Sea, Greece. In total, 756 individuals were collected, Emmanouil Kouroupakis by artisanal net fishery and log transformed data were used at the (L-W) relationships assessment. Three (1) Marine Sciences Department, body parts were measured [carapace length (CL), abdominal length (ABL), and telson width (TW)]. Both School of the Environment, females and males demonstrated similarities at their allometric profiles except for CL-W, where females University of the Aegean, present positive allometric profile and males negative. The present findings are mostly in accordance Mytilene, Lesvos Island, Greece (2) Hellenic Centre for Marine with earlier studies from Mediterranean. Research, Institute of Marine Biology, Biotechnology and Keywords: Squilla mantis, stomatopoda, allometric growth, fisheries, Aegean Sea, east Mediterranean Aquaculture, Agios Kosmas, Sea Hellenikon, Athens, Greece 1. Introduction Marianna Lazaridou Squilla mantis (Linnaeus, 1758), spot-tail mantis shrimp, belongs to the Order Stomatopoda, 18, Armpani str. -

Quality Changes During Frozen Storage of Mechanical-Separated Flesh Obtained from an Underutilized Crustacean
foods Article Quality Changes during Frozen Storage of Mechanical-Separated Flesh Obtained from an Underutilized Crustacean Silvia Tappi 1,2,*, Ana Cristina De Aguiar Saldanha Pinheiro 1 , Dario Mercatante 3 , Gianfranco Picone 1 , Francesca Soglia 1 , Maria Teresa Rodriguez-Estrada 2,3, Massimiliano Petracci 1 , Francesco Capozzi 1,2 and Pietro Rocculi 1,2 1 Department of Agricultural and Food Sciences, Alma Mater Studiorum—University of Bologna, 47521 Cesena, Italy; [email protected] (A.C.D.A.S.P.); [email protected] (G.P.); [email protected] (F.S.); [email protected] (M.P.); [email protected] (F.C.); [email protected] (P.R.) 2 Interdepartmental Centre for Agri-Food Industrial Research, Alma Mater Studiorum— University of Bologna, (FC) 47521 Cesena, Italy; [email protected] 3 Department of Agricultural and Food Sciences, Alma Mater Studiorum—University of Bologna, 40127 Bologna, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0547-338103 Received: 22 September 2020; Accepted: 15 October 2020; Published: 17 October 2020 Abstract: Despite their high nutritional value, high quantities of fish caught in the Adriatic Sea are underused or discarded for their insignificant economic value. Mechanical separation of flesh represents an opportunity for developing innovative semi-finished products, even if it can promote an increased quality degradation rate. The aim of this study was to evaluate physico-chemical modifications of mechanically separated mantis shrimp flesh during deep-freezing storage. Flesh samples obtained using a belt-drum separator, frozen and vacuum-packed, were stored at 3 temperatures (industrial: 26 C; domestic: 18 C and abuse: 10 C) for 12 months. -

Mantis Shrimp - Wikipedia
Mantis shrimp - Wikipedia https://en.wikipedia.org/wiki/Mantis_shrimp Mantis shrimp Mantis shrimps , or stomatopods , are marine crustaceans of the Mantis shrimp order Stomatopoda . Some species have specialised calcified "clubs" that can strike with great power, while others have sharp forelimbs used Temporal range: 400–0 Ma to capture prey. They branched from other members of the class Pre Є Є O S D C P T J K Pg N Malacostraca around 340 million years ago. [2] Mantis shrimps typically grow to around 10 cm (3.9 in) in length. A few can reach up to 38 cm (15 in). [3] The largest mantis shrimp ever caught had a length of 46 cm (18 in); it was caught in the Indian River near Fort Pierce, Florida, in the United States.[4] A mantis shrimp's carapace (the bony, thick shell that covers crustaceans and some other species) covers only the rear part of Odontodactylus scyllarus the head and the first four segments of the thorax. Varieties range from shades of brown to vivid colors, as more than 450 species of mantis Scientific classification shrimps are known. They are among the most important predators in Kingdom: Animalia many shallow, tropical and subtropical marine habitats. However, Phylum: Arthropoda despite being common, they are poorly understood, as many species spend most of their lives tucked away in burrows and holes. [5] Subphylum: Crustacea Called "sea locusts" by ancient Assyrians, "prawn killers" in Australia, [6] Class: Malacostraca and now sometimes referred to as "thumb splitters"—because of the Subclass: Hoplocarida [7] animal's ability to inflict painful gashes if handled incautiously Order: Stomatopoda —mantis shrimps have powerful claws that are used to attack and kill Latreille, 1817 prey by spearing, stunning, or dismembering. -

Why Protect Decapod Crustaceans Used As Models in Biomedical Research and in Ecotoxicology? Ethical and Legislative Considerations
animals Article Why Protect Decapod Crustaceans Used as Models in Biomedical Research and in Ecotoxicology? Ethical and Legislative Considerations Annamaria Passantino 1,* , Robert William Elwood 2 and Paolo Coluccio 3 1 Department of Veterinary Sciences, University of Messina-Polo Universitario Annunziata, 98168 Messina, Italy 2 School of Biological Sciences, Queen’s University, Belfast BT9 5DL, Northern Ireland, UK; [email protected] 3 Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA), University of Florence, Viale Pieraccini 6, 50139 Firenze, Italy; paolo.coluccio@unifi.it * Correspondence: [email protected] Simple Summary: Current European legislation that protects animals used for scientific purposes excludes decapod crustaceans (for example, lobster, crab and crayfish) on the grounds that they are non-sentient and, therefore, incapable of suffering. However, recent work suggests that this view requires substantial revision. Our current understanding of the nervous systems and behavior of decapods suggests an urgent need to amend and update all relevant legislation. This paper examines recent experiments that suggest sentience and how that work has changed current opinion. It reflects on the use of decapods as models in biomedical research and in ecotoxicology, and it recommends that these animals should be included in the European protection legislation. Abstract: Decapod crustaceans are widely used as experimental models, due to their biology, their sensitivity to pollutants and/or their convenience of collection and use. Decapods have been Citation: Passantino, A.; Elwood, viewed as being non-sentient, and are not covered by current legislation from the European Par- R.W.; Coluccio, P. Why Protect liament. However, recent studies suggest it is likely that they experience pain and may have the Decapod Crustaceans Used as capacity to suffer. -

APP Environmental and Social Sustainability Report for Indonesia
2007 Growing a Sustainable Future Environmental and Social Sustainability Report for Indonesia TABLE OF CONTENTS APP’s Fiber Supply Appendices 103 Introduction 124 Appendix I: and Overview Governance Structure 103 Sustainable Forest 127 Appendix II: Management Operational Structure 105 APP’s Sustainable 128 Appendix III: Fiber Supply Association Memberships 2 Report Scope 106 Environmental 129 Appendix IV: 4 Message from Performance Indonesia’s Units of Governance the Chairman 111 Social Performance 130 Appendix V: Policy Documents 132 Appendix VI: Audit Statement Summaries 134 Appendix VII: Corporate Overview APP’s Conservation Stakeholder Interviews Initiatives for this Report 135 Appendix VIII: 6 Introduction to APP APP Product 8 APP’s Vision 116 Overview Environmental Credentials 8 Corporate Governance 116 APP Conservation Value 136 Appendix IX: Toolkit for Indonesia Plant Variety Protection 9 Sustainability and Certificate for Eucalyptus Corporate Strategy 117 Giam Siak Kecil – Bukit Batu Biosphere Reserve Pellita 05 (EP05) 13 APP’s Fiber Sources 118 Taman Raja 137 Appendix X: 14 APP’s Relationship Conservation Area Conservation Initiative with Stakeholders Working Group Members 118 Senepis Buluhala Tiger Sanctuary 139 Appendix XI: IUCN Red List Species 119 Kutai Orangutan From Conservation Conservation Group Mill Performance Value Forest Survey 141 Appendix XII: IUCN Red List Species - 18 Introduction and Overview 121 Glossary Bukit Batu Biosphere 19 Mills at a Glance Reserve 19 Products 142 Appendix XIII: IUCN Red List Species -