Femoral Neck System Surgical Technique Image Intensifier Control
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RICE, CARL ROSS. Diocletian's “Great
ABSTRACT RICE, CARL ROSS. Diocletian’s “Great Persecutions”: Minority Religions and the Roman Tetrarchy. (Under the direction of Prof. S. Thomas Parker) In the year 303, the Roman Emperor Diocletian and the other members of the Tetrarchy launched a series of persecutions against Christians that is remembered as the most severe, widespread, and systematic persecution in the Church’s history. Around that time, the Tetrarchy also issued a rescript to the Pronconsul of Africa ordering similar persecutory actions against a religious group known as the Manichaeans. At first glance, the Tetrarchy’s actions appear to be the result of tensions between traditional classical paganism and religious groups that were not part of that system. However, when the status of Jewish populations in the Empire is examined, it becomes apparent that the Tetrarchy only persecuted Christians and Manichaeans. This thesis explores the relationship between the Tetrarchy and each of these three minority groups as it attempts to understand the Tetrarchy’s policies towards minority religions. In doing so, this thesis will discuss the relationship between the Roman state and minority religious groups in the era just before the Empire’s formal conversion to Christianity. It is only around certain moments in the various religions’ relationships with the state that the Tetrarchs order violence. Consequently, I argue that violence towards minority religions was a means by which the Roman state policed boundaries around its conceptions of Roman identity. © Copyright 2016 Carl Ross Rice All Rights Reserved Diocletian’s “Great Persecutions”: Minority Religions and the Roman Tetrarchy by Carl Ross Rice A thesis submitted to the Graduate Faculty of North Carolina State University in partial fulfillment of the requirements for the degree of Master of Arts History Raleigh, North Carolina 2016 APPROVED BY: ______________________________ _______________________________ S. -
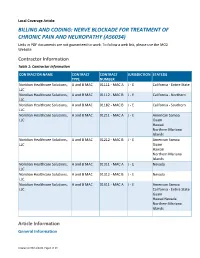
BILLING and CODING: NERVE BLOCKADE for TREATMENT of CHRONIC PAIN and NEUROPATHY (A56034) Links in PDF Documents Are Not Guaranteed to Work
Local Coverage Article: BILLING AND CODING: NERVE BLOCKADE FOR TREATMENT OF CHRONIC PAIN AND NEUROPATHY (A56034) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Table 1: Contractor Information CONTRACTOR NAME CONTRACT CONTRACT JURISDICTION STATE(S) TYPE NUMBER Noridian Healthcare Solutions, A and B MAC 01111 - MAC A J - E California - Entire State LLC Noridian Healthcare Solutions, A and B MAC 01112 - MAC B J - E California - Northern LLC Noridian Healthcare Solutions, A and B MAC 01182 - MAC B J - E California - Southern LLC Noridian Healthcare Solutions, A and B MAC 01211 - MAC A J - E American Samoa LLC Guam Hawaii Northern Mariana Islands Noridian Healthcare Solutions, A and B MAC 01212 - MAC B J - E American Samoa LLC Guam Hawaii Northern Mariana Islands Noridian Healthcare Solutions, A and B MAC 01311 - MAC A J - E Nevada LLC Noridian Healthcare Solutions, A and B MAC 01312 - MAC B J - E Nevada LLC Noridian Healthcare Solutions, A and B MAC 01911 - MAC A J - E American Samoa LLC California - Entire State Guam Hawaii Nevada Northern Mariana Islands Article Information General Information Created on 05/11/2021. Page 1 of 23 Article ID A56034 Article Title Billing and Coding: Nerve Blockade for Treatment of Chronic Pain and Neuropathy Article Type Billing and Coding AMA CPT / ADA CDT / AHA NUBC Copyright Statement CPT codes, descriptions and other data only are copyright 2020 American Medical Association. All Rights Reserved. Applicable FARS/HHSARS apply. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. -

Journeys to Byzantium? Roman Senators Between Rome and Constantinople
Journeys to Byzantium? Roman Senators Between Rome and Constantinople Master’s Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Arts in the Graduate School of The Ohio State University By Michael Anthony Carrozzo, B.A Graduate Program in History The Ohio State University 2010 Thesis Committee: Kristina Sessa, Advisor Timothy Gregory Anthony Kaldellis Copyright by Michael Anthony Carrozzo 2010 Abstract For over a thousand years, the members of the Roman senatorial aristocracy played a pivotal role in the political and social life of the Roman state. Despite being eclipsed by the power of the emperors in the first century BC, the men who made up this order continued to act as the keepers of Roman civilization for the next four hundred years, maintaining their traditions even beyond the disappearance of an emperor in the West. Despite their longevity, the members of the senatorial aristocracy faced an existential crisis following the Ostrogothic conquest of the Italian peninsula, when the forces of the Byzantine emperor Justinian I invaded their homeland to contest its ownership. Considering the role they played in the later Roman Empire, the disappearance of the Roman senatorial aristocracy following this conflict is a seminal event in the history of Italy and Western Europe, as well as Late Antiquity. Two explanations have been offered to explain the subsequent disappearance of the Roman senatorial aristocracy. The first involves a series of migrations, beginning before the Gothic War, from Italy to Constantinople, in which members of this body abandoned their homes and settled in the eastern capital. -

And Rome's Legacies
Christianity AND ROME’S LEGACIES Old Religions New Testament MARK MAKES HIS MARK NOT SO SIMPLE TEMPLES IN PARTNERSHIP WITH christianity_FC.indd 1 3/6/17 3:32 PM 2 Religions in Rome The earliest Romans saw their gods as spirits or powerful forces of nature. These gods did not have personalities or emotions or act in any other way like human beings. However, as Rome began to build an empire, the Romans were exposed to new ideas. Through contact with the Greeks, the Romans’ idea of gods and goddesses changed. The Greeks believed in gods and god- desses who behaved very much like human beings. Their gods could be jeal- ous, angry, passionate, kind, foolish, or petty. The Romans borrowed this idea u THE ROMANS People did not go to and honey, burned honored their gods a temple to worship sweet-smelling from the Greeks. They even borrowed by building temples. the god. Rather, a incense, and sac- some of the Greek gods and goddesses. Inside each temple temple was where rificed animals to No longer were the Roman gods spir- was a statue of a priests made honor the god. god or goddess. offerings of cakes its or forces of nature. They were now divine and human at the same time. u UNTIL THE in private people 300s CE, the Roman were free to think u THE ROMANS wisdom. During festival day, priests ticular, no legal religion was a and say what they honored their gods Cerealia, Romans performed rituals work was allowed. state religion. wanted to. Over with more than 100 honored the god- and sacrifices Celebrations includ- The emperor was time, the emperor festivals every year. -

Reconstructive
RECONSTRUCTIVE Angiosomes of the Foot and Ankle and Clinical Implications for Limb Salvage: Reconstruction, Incisions, and Revascularization Christopher E. Attinger, Background: Ian Taylor introduced the angiosome concept, separating the M.D. body into distinct three-dimensional blocks of tissue fed by source arteries. Karen Kim Evans, M.D. Understanding the angiosomes of the foot and ankle and the interaction among Erwin Bulan, M.D. their source arteries is clinically useful in surgery of the foot and ankle, especially Peter Blume, D.P.M. in the presence of peripheral vascular disease. Paul Cooper, M.D. Methods: In 50 cadaver dissections of the lower extremity, arteries were injected Washington, D.C.; New Haven, with methyl methacrylate in different colors and dissected. Preoperatively, each Conn.; and Millburn, N.J. reconstructive patient’s vascular anatomy was routinely analyzed using a Dopp- ler instrument and the results were evaluated. Results: There are six angiosomes of the foot and ankle originating from the three main arteries and their branches to the foot and ankle. The three branches of the posterior tibial artery each supply distinct portions of the plantar foot. The two branches of the peroneal artery supply the anterolateral portion of the ankle and rear foot. The anterior tibial artery supplies the anterior ankle, and its continuation, the dorsalis pedis artery, supplies the dorsum of the foot. Blood flow to the foot and ankle is redundant, because the three major arteries feeding the foot have multiple arterial-arterial connections. By selectively performing a Doppler examination of these connections, it is possible to quickly map the existing vascular tree and the direction of flow. -

Granby Mother Kills Her 2 Small Children
r"T ' ' j •'.v> ■ I SATUBDAY, DECEMBER 24, 1960 V‘‘‘ iOanrtifBter E w ttins $?raUt Ifii'J •• t Not (me of the men MW anything funny In the process. No Herald Social Worker About To>m Heard Along Main Street! Just like a woman 7 Some of the men fall in the know-how Pepart- OnMdhday Sought by Town WATBS wlU meet And on Some o) Manehe$ter*§ Side SlreeU,Too ment, too, when it. comes to freeing l^iesdey evening nt the Italian mechanical horses from Ice traps. The town is accepting appUca- The Manchester Evening tiona through Wednesday to fill Amertean ' Chib on Sldrltlge St. <^been a. luncheon guest at the inn. 1 0 :' Welgbtog In win be from 7 to 8 What Are You Gonna Do? It ' So Reverse It a social work^ post with sn an One Herald aubacriber is very Lt. Leander, who is stationed at Herald will-not publish Mon 'Words of advice wer^ voiced nual salary range of 84,004 to YOL. LXXX, NO. 7S (SIXTEEN PAGES) MANCHESTER, CONN„ TUESDAY. DECEMBER 27, 1960 (ChuwUtsg AgvsrttalBg m Fag» 14) PRICE PIVB V-*A red-faced becauee her 10-year-oM the U.S. Navy atatipn .gt Orange, irom one local youngster to an day, the day after Christmas, 14,914 and an allowance for use son got carried away with the Tex., had spent the afterhiibh vla- other as they walked along the Merty Christmas to all. of the worker's own car. Handd S. Crozler will show Christmas spirit. Iting a state park, San Jacinto street in the ijear zero weather. -

The Political and Military Aspects of Accession of Constantine the Great
Graeco-Latina Brunensia 24 / 2019 / 2 https://doi.org/10.5817/GLB2019-2-2 The Political and Military Aspects of Accession of Constantine the Great Stanislav Doležal (University of South Bohemia in České Budějovice) Abstract The article argues that Constantine the Great, until he was recognized by Galerius, the senior ČLÁNKY / ARTICLES Emperor of the Tetrarchy, was an usurper with no right to the imperial power, nothwithstand- ing his claim that his father, the Emperor Constantius I, conferred upon him the imperial title before he died. Tetrarchic principles, envisaged by Diocletian, were specifically put in place to supersede and override blood kinship. Constantine’s accession to power started as a military coup in which a military unit composed of barbarian soldiers seems to have played an impor- tant role. Keywords Constantine the Great; Roman emperor; usurpation; tetrarchy 19 Stanislav Doležal The Political and Military Aspects of Accession of Constantine the Great On 25 July 306 at York, the Roman Emperor Constantius I died peacefully in his bed. On the same day, a new Emperor was made – his eldest son Constantine who had been present at his father’s deathbed. What exactly happened on that day? Britain, a remote province (actually several provinces)1 on the edge of the Roman Empire, had a tendency to defect from the central government. It produced several usurpers in the past.2 Was Constantine one of them? What gave him the right to be an Emperor in the first place? It can be argued that the political system that was still valid in 306, today known as the Tetrarchy, made any such seizure of power illegal. -

The Edictum Theoderici: a Study of a Roman Legal Document from Ostrogothic Italy
The Edictum Theoderici: A Study of a Roman Legal Document from Ostrogothic Italy By Sean D.W. Lafferty A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Department of History University of Toronto © Copyright by Sean D.W. Lafferty 2010 The Edictum Theoderici: A Study of a Roman Legal Document from Ostrogothic Italy Sean D.W. Lafferty Doctor of Philosophy Department of History University of Toronto 2010 Abstract This is a study of a Roman legal document of unknown date and debated origin conventionally known as the Edictum Theoderici (ET). Comprised of 154 edicta, or provisions, in addition to a prologue and epilogue, the ET is a significant but largely overlooked document for understanding the institutions of Roman law, legal administration and society in the West from the fourth to early sixth century. The purpose is to situate the text within its proper historical and legal context, to understand better the processes involved in the creation of new law in the post-Roman world, as well as to appreciate how the various social, political and cultural changes associated with the end of the classical world and the beginning of the Middle Ages manifested themselves in the domain of Roman law. It is argued here that the ET was produced by a group of unknown Roman jurisprudents working under the instructions of the Ostrogothic king Theoderic the Great (493-526), and was intended as a guide for settling disputes between the Roman and Ostrogothic inhabitants of Italy. A study of its contents in relation to earlier Roman law and legal custom preserved in imperial decrees and juristic commentaries offers a revealing glimpse into how, and to what extent, Roman law survived and evolved in Italy following the decline and eventual collapse of imperial authority in the region. -

Barbarians Invade Rome! Questions
But the power of Rome was weakening. By the late 400s, Rome was Name no longer the mighty power that it had once been. In 476, the Hun leader, Odoacer, seized power in the western half of the Roman Empire and declared himself king of Italy. Barbarians Invade Rome! The eastern half of the Roman Empire tried to take power back in the west by sending troops, under the leadership of King Theodoric By Sharon Fabian of the Ostrogoths, to fight Odoacer. The Ostrogoths killed Odoacer, and Theodoric became the new leader of Italy. The Roman Empire, at its height, extended across much of Europe, but even then there were other groups of people who were The Roman Empire in the east continued, but Roman rule in the not part of the Roman Empire living in Europe too. Many of these west had come to an end. Europe entered into an era of uncertainty. groups, called tribes, lived in the far north. Others lived in various Rulers changed frequently, as one leader attacked and defeated parts of Europe not occupied by the Roman Empire. another. Invasions, attacks, and feuds were commonplace. It was the beginning of the Middle Ages. The barbarian tribes, as many of them were known, didn't like the idea of settling down and farming. They preferred a roaming, Today, when we hear of Huns, we picture wild-eyed, long-haired, warlike lifestyle. Due to climate changes and other factors, many of screaming invaders. the tribes began to migrate closer to the Roman Empire and sometimes even settle within the borders of the empire. -

Merovingian Queens: Status, Religion, and Regency
Merovingian Queens: Status, Religion, and Regency Jackie Nowakowski Honors Thesis Submitted to the Department of History, Georgetown University Advisor: Professor Jo Ann Moran Cruz Honors Program Chair: Professor Alison Games May 4, 2020 Nowakowski 1 Table of Contents: Acknowledgments………………………………………………………………………………..2 Map, Genealogical Chart, Glossary……………………………………………………………3 Introduction………………………………………………………………………………………7 Chapter 1: The Makings of a Merovingian Queen: Slave, Concubine, or Princess………..18 Chapter 2: Religious Authority of Queens: Intercessors and Saints………………………..35 Chapter 3: Queens as Regents: Scheming Stepmothers and Murdering Mothers-in-law....58 Conclusion……………………………………………………………………………………....80 Bibliography…………………………………………………………………………………….83 Nowakowski 2 Acknowledgements I would like to thank Professor Moran Cruz for all her guidance and advice; you have helped me become a better scholar and writer. I also want to thank Professor Games for your constant enthusiasm and for creating a respectful and fun atmosphere for our seminar. Your guidance over these past two semesters have been invaluable. I am also so grateful for my classmates, who always gave me honest and constructive feedback; I have enjoyed seeing where your projects take you. Most of all, I would like to thank my family and friends for listening to me talk nonstop about a random, crazy, dysfunctional family from the sixth century. I am incredibly thankful for my parents, sister, and friends for their constant support. Thank you mom for listening to a podcast on the Merovingians so you could better understand what I am studying. You have always inspired me to work hard and I probably wouldn’t have written a thesis without you as my inspiration. I also want to thank my dad, who always supported my studies and pretended to know more about a topic than he actually did. -

Jordanes and the Invention of Roman-Gothic History Dissertation
Empire of Hope and Tragedy: Jordanes and the Invention of Roman-Gothic History Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Brian Swain Graduate Program in History The Ohio State University 2014 Dissertation Committee: Timothy Gregory, Co-advisor Anthony Kaldellis Kristina Sessa, Co-advisor Copyright by Brian Swain 2014 Abstract This dissertation explores the intersection of political and ethnic conflict during the emperor Justinian’s wars of reconquest through the figure and texts of Jordanes, the earliest barbarian voice to survive antiquity. Jordanes was ethnically Gothic - and yet he also claimed a Roman identity. Writing from Constantinople in 551, he penned two Latin histories on the Gothic and Roman pasts respectively. Crucially, Jordanes wrote while Goths and Romans clashed in the imperial war to reclaim the Italian homeland that had been under Gothic rule since 493. That a Roman Goth wrote about Goths while Rome was at war with Goths is significant and has no analogue in the ancient record. I argue that it was precisely this conflict which prompted Jordanes’ historical inquiry. Jordanes, though, has long been considered a mere copyist, and seldom treated as an historian with ideas of his own. And the few scholars who have treated Jordanes as an original author have dampened the significance of his Gothicness by arguing that barbarian ethnicities were evanescent and subsumed by the gravity of a Roman political identity. They hold that Jordanes was simply a Roman who can tell us only about Roman things, and supported the Roman emperor in his war against the Goths. -

Introduction, Part 2: Build-Out & Property Owners
Introduction, Part 2 -- 1 Introduction, Part 2: Build-Out & Property Owners complied by John Lofland Part 2 of the Introduction describes the “E-5-00s” from three vantage points: The first is the pace at which the 16 main homes were built over four decades; The second is the time-space clusters of the 16 homes. And the third is ownership of properties as shown on City-issued maps. l. PACE. The term “pace” refers to the rate at which the 16 main houses were constructed decade-by-decade. This topic is of interest because of its sharp contrast with the pace of housing construction in neighborhoods in more recent times. More recently, all the houses on a single block go up pretty much at the same time. In that way, housing tracts are rather like mushrooms patches. Nothing is there one day, but there is a patch the next day. The 16 main E-500 homes were constructed in four different decades, the 1910s, ‘20s, ‘30s, and ‘40s. No homes were there about 1910. (Three enumerated in 1905 do not appear in later records and likely burned down.) and none were built after 1950 (after 1946, to be exact). The 16 were not, though, built at an even pace across the four decades (i.e., four a decade). Instead, there was a slow start (one built in the 1910s), a small rise in the 1920s (four built), a rapid rise in the 1930s (ten built), and “build out” in the 1940s (one built). Here are the details of which houses were built when.