Changing Fire Regimes in Tropical and Subtropical Australia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
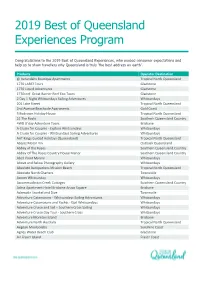
2019 Best of Queensland Experiences Program
2019 Best of Queensland Experiences Program Congratulations to the 2019 Best of Queensland Experiences, who exceed consumer expectations and help us to show travellers why Queensland is truly ‘the best address on earth’. Products Operator Destination @ Verandahs Boutique Apartments Tropical North Queensland 1770 LARC! Tours Gladstone 1770 Liquid Adventures Gladstone 1770reef Great Barrier Reef Eco Tours Gladstone 2 Day 1 Night Whitsundays Sailing Adventures Whitsundays 201 Lake Street Tropical North Queensland 2nd Avenue Beachside Apartments Gold Coast 3 Bedroom Holiday House Tropical North Queensland 31 The Rocks Southern Queensland Country 4WD G'day Adventure Tours Brisbane A Cruise for Couples - Explore Whitsundays Whitsundays A Cruise for Couples - Whitsundays Sailing Adventures Whitsundays AAT Kings Guided Holidays (Queensland) Tropical North Queensland Abajaz Motor Inn Outback Queensland Abbey of the Roses Southern Queensland Country Abbey Of The Roses Country House Manor Southern Queensland Country Abell Point Marina Whitsundays Above and Below Photography Gallery Whitsundays Absolute Backpackers Mission Beach Tropical North Queensland Absolute North Charters Townsville Accom Whitsunday Whitsundays Accommodation Creek Cottages Southern Queensland Country Adina Apartment Hotel Brisbane Anzac Square Brisbane Adrenalin Snorkel and Dive Townsville Adventure Catamarans - Whitsundays Sailing Adventures Whitsundays Adventure Catamarans and Yachts - ISail Whitsundays Whitsundays Adventure Cruise and Sail – Southern Cross Sailing Whitsundays -

Study English in Tropical Queensland, Australia Gain the Language Skills You Need for Success Darwin
Study English in Tropical Queensland, Australia Gain the language skills you need for success Darwin Cairns campus Townsville campus Brisbane Perth Sydney Canberra Adelaide Melbourne Hobart Getting to JCU Australia Cairns Cairns is easy to travel to, with direct flights to and from Japan, Hong Kong, Singapore, New Zealand, Papua New Guinea, the Philippines and Guam, with seasonal flights to Shanghai. There are direct flights to popular Australian cities including Sydney, Melbourne, Brisbane, the Gold Coast, Perth, Darwin, and Townsville. Townsville Townsville is a busy regional airport with connections to most Australian cities and direct flights to Bali. We now have a direct flight from Papua New Guinea. We recommend that new students take one of the regular 50 minute flights from Cairns to Townsville, as JCU Townsville is a four hour drive from Cairns Airport and more than a 16 hour drive from Brisbane. Apply now Website: jcu.edu.au/studyenglish Email: [email protected] Phone: +61 7 4781 5601 This publication is intended as a general introduction to James Cook University Study Tours. All care has been taken to ensure information is correct at the time of printing. The University reserves the right to alter any information without prior notice. CRICOS PROVIDER CODE: 00117J CRICOS PROVIDER Welcome to James Cook University About James Cook University Study English in Tropical Queensland, Australia Ranked in the top 2 per cent of universities worldwide*, James Cook University is Australia’s The communities of Cairns and Townsville leading university for the Tropics. Offering would like to welcome you. Both cities are located in beautiful natural environments, excellence in teaching and research, over 22,000 with lots of free outdoor activities, and students, including 7,500 international students access to world-class tourist destinations, from over 100 countries, study for JCU degrees. -

Magnetic Island Townsville &
Cairns Townsville Airlie Beach Rockhampton Brisbane Townsville & Magnetic Island NORTH QUEENSLAND, AUSTRALIA Cairns Townsville Airlie Beach Charters Towers DRIVE TIMES 4hrs Townsville to Cairns Sunshine Coast 3hrs Townsville to Airlie Beach Brisbane 1.5hrs Townsville to Charters Towers Photo courtesy of @wheres yo yo the hidden secret of North Queensland sealinkqld.com.au WELCOME to North Queensland Townsville, the capital of North Queensland, is a vibrant and bustling city surrounded by iconic Australian landscapes of tropical islands, golden beaches, rainforest, wetlands and the outback. Featuring an international airport and national rail connections, it has never been easier to experience life in the tropics! Dive the Yongala Wreck, visit Reef HQ The Ville Resort & Casino Aquarium the world’s largest living coral reef aquarium, join a platypus safari in Paluma or just relax along Townsville’s waterfront precinct The Strand. There are experiences to suit everyone! Townsville is the events capital of North Queensland, with an exciting calendar of sporting, lifestyle, music and marine events showcased each year. Classique B&B Quest on Eyre Reef HQ Wallaman Falls 3 NIGHT TASTE OF TOWNSVILLE 2 NIGHT RAINFOREST SELF DRIVE INCLUSIONS INCLUSIONS • 3 Nights’ Accommodation, Townsville • 2 Nights’ Accommodation, • Tropic Highlights Package Hidden Valley Rustic Cabin – Return ferry transfers • Platypus & solar tour – All day bus pass on Magnetic Island • Guided Nocturnal Night walk – Entry into Bungalow Bay Koala Village, • Hot Breakfast daily Magnetic Island • Self-guided maps of the – Entry into Reef HQ and Museum of Hidden Valley/Paluma area Tropical Queensland in Townsville Hidden Valley Cabins • Townsville Military & Scenic Tour DESCRIPTION DESCRIPTION This self-drive escape is perfect for those seeking Discover the beauty, culture and history of one of the to retreat to a natural setting. -

Far North Queensland
etropic 8 (2009): Flick, Caltabiano, & Bentrupperbaumer, Far North Queensland Far North Queensland Flick, Brigitta1, Nerina Caltabiano2, Joan Bentrupperbäumer3 Department of Psychology, James Cook University, PO Box 6811, Cairns QLD 4879, Australia, [email protected],1 Department of Psychology, James Cook University , [email protected],2 School of Earth and Environmental Sciences, James Cook University, [email protected] ABSTRACT Landowners (100 males and 21 females) within the catchment of the Great Barrier Reef Lagoon located between the Barron and the South Johnstone Rivers responded to a postal survey assessing attitudes towards their streamside forests. 121 useable questionnaires were used to identify the underlying components within landowner's attitudes. Factor analysis revealed two components, namely, awareness of human impact on local streamside forest, and appreciation of environmental benefits and functions of streamside forest. Only factor 2, appreciation of environment benefits and functions of streamside forest, was found to significantly predict good streamside forest management practices as self-reported by landowners. There is a need to understand the human impact on streamside zones. Far North Queensland is an area of global biological significance. It contains two World Heritage areas that were listed based on natural attributes alone and fulfilled all four World Heritage criteria. The Great Barrier Reef (GBR) was declared World Heritage in 1981 and the Wet Tropics in 1988 (World Heritage List, 2009) . The lowlands of the GBR Catchment in the study area are comprised almost entirely of private land that is largely farmed and urbanised. In the course of time since European settlement much of the native vegetation was cleared, especially the streamside forests were diminished or destroyed (Productivity Commission, 2003). -

We Would Rather Be Ruined Than Changed We Would Rather Die in Our Dread Than Climb the Cross of the Moment and Let Our Illusions Die W.H
SOME EARLY ILLUSIONS CONCERNING NORTH QUEENSLAND Ray Sumner Department of Geography James Cook University of North Queensland We would rather be ruined than changed We would rather die in our dread Than climb the cross of the moment And let our illusions die W.H. Auden Our assessment of any landscape results as much from how we view it as from the reality of what is actually there. As Brookfield said "decision makers operating in an environment base their decisions on the environ- ment as they perceive it, not as it is". 1 The Europeans who explored tropical Queensland entered an unknown land which they were required to examine and then offer an assessment of its potential. Since the environment confronted the explorers with a situation of complete uncertainty, a subjective error component was inevitable in their description and analysis, but in fact their reaction to the new environment was affected by what they wanted to see, or thought they saw, as much as by what was actually there. The image of new country recounted by each explorer resulted largely from his response to visual stimuli in the new environment. Since observation and interpretation are enhanced by some degree of familiarity, a history of prior exploration in the south might be expected to improve the performance of explorers in the Tropics, but this was no criterion for an objective appraisal of the new areas. After three successful journeys of exploration in southern states, the Surveyor-General Major (later Sir) Thomas Mitchell concluded his trip to central Queensland with a spectacular blunder; Edmund Kennedy had a background of inland journeys, but died in a disastrous attempt on Cape York. -

Queensland Rail Holidays
QUEENSLAND RAIL HOLIDAYS 2021/22 QUEENSLAND RAIL HOLIDAYS Sunlover Holidays loves Queensland and you will too. Discover the beautiful coastline and marvel at spectacular outback landscapes as you traverse Queensland on an unforgettable rail holiday. We have combined a great range of accommodation and tour options with these iconic rail journeys to create your perfect holiday. Whether you’re planning a short break, a romantic getaway or the ultimate family adventure, Sunlover Holidays can tailor-make your dream holiday. Use this brochure for inspiration, then let our travel experts assist you to experience Queensland your way – happy travelling! Front cover image: Spirit of Queensland Image this page: Tilt Train Enjoy hearty Outback inspired cuisine and unparalleled service on the Spirit of the Outback CONTENTS Planning Your Rail Holiday 5 COASTAL RAIL 6 Tilt Train 7 Tilt Train Holiday Packages 8 Spirit of Queensland 10 Spirit of Queensland Holiday Packages 12 Kuranda Scenic Railway 17 GULF SAVANNAH RAIL 18 Lawn Hill Gorge, Queensland Gulflander 19 Gulf Savannah Holiday Packages 20 OUTBACK RAIL 23 Spirit of the Outback 24 Ultimate Outback Queensland Adventure – Fully Escorted 26 Spirit of the Outback Holiday Packages 28 Westlander 33 Westlander Holiday Packages 33 Inlander 34 Inlander Holiday Packages 34 Booking Conditions 35 Michaelmas Cay, Tropical North Queensland Valid 1 April 2021 – 31 March 2022 3 Take the track less travelled onboard the Inlander Thursday Island Weipa Cooktown Green Island Kuranda Cairns Karumba Normanton Tully -

Tropical Health and Biodiscovery
TROPICAL HEALTH AND BIODISCOVERY Queensland is in a strong position to contribute to Queensland’s unique tropical biodiversity, world- solutions for current and emerging challenges in class biotechnology research and development tropical health and medicine. Most of the world’s capabilities and supportive legislative environment population will live in a tropical climate by 2050. provides significant opportunities that can be There are outstanding opportunities here for harnessed for the discovery and development of Queensland and Australian tropical medicine given innovative solutions to tropical health challenges Queensland’s tropical location and our expertise in and novel natural products for drug development. tropical and emerging diseases. Our location also Australia is one of only 17 countries recognised to provides easy access to the fast-growing nations of have extraordinarily high levels of biodiversity or the Asia–Pacific region, who seek solutions to long- ‘megadiversity’. These 17 countries have less than standing tropical health issues. 10% of the global surface, but support more than Queensland has a comparative advantage in tropical 70% of the biological diversity on earth. Australia health and medicine expertise through its position is the only megadiverse nation on earth that is a as a developed economy spanning a wide variety of developed nation with relatively low population tropical zones, and its underpinning excellence in density. Moreover, Queensland is Australia’s tropical health and medicine research. For example, most biodiverse state, with 13 terrestrial and 14 the Australian Institute of Tropical Health and marine bioregions supporting more than 1000 Medicine is Australia’s dedicated tropical health types of ecosystem. The Biodiscovery Act 2004 and medical research institute. -

Tropical Coast National Parks Contents Parks at a Glance
Queensland National Parks Tropical Coast national parks Contents Parks at a glance .........................................................................2 Girringun National Park .............................................................14 Welcome .....................................................................................3 Hinchinbrook Island National Park ............................................16 Map of the Tropical Coast national parks ....................................4 Girramay National Park .............................................................18 Plan your getaway .......................................................................6 Tully Gorge National Park ..........................................................19 Wet Tropics World Heritage Area ................................................6 Djiru and Clump Mountain national parks .................................20 Great Barrier Reef World Heritage Area ........................................6 Eubenangee Swamp and Russell River national parks ..............21 Choose your adventure ...............................................................8 Wooroonooran National Park ....................................................22 Bowling Green Bay National Park ................................................9 Smithfield and Mount Whitfield conservation parks .................24 Townsville Town Common and Tropical island national parks ...................................................25 Cape Pallarenda conservation parks .........................................10 -

The Importance of Protecting and Conserving the Wet Tropics
The Importance of Protecting and Conserving the Wet Tropics Synthesis of NERP Tropical Ecosystems Hub Tropical Rainforest Research Outputs 2011-2014 Compiled by RRRC The Importance of Protecting and Conserving the Wet Tropics A Synthesis of NERP Tropical Ecosystems Hub Tropical Rainforest Research Outputs 2011-2014 Julie Carmody, Helen Murphy, Ro Hill, Carla Catterall, Steve Goosem, Allan Dale, David Westcott, Justin Welbergen, Luke Shoo, Natalie Stoeckl, Michelle Esparon Supported by the Australian Government’s National Environmental Research Program © Reef and Rainforest Research Centre Limited National Library of Australia Cataloguing-in-Publication entry: 978-1-925088-60-1 This report should be cited as: Carmody, J., Murphy, H., Hill, R., Catterall, C., Goosem, S., Dale, A., Westcott, D., Welbergen, J., Shoo, L., Stoeckl, N., Esparon, M. (2015) The Importance of Protecting and Conserving the Wet Tropics: A synthesis of NERP Tropical Ecosystems Hub Tropical Rainforest Outputs 2011-2014. Report to the National Environmental Research Program. Reef and Rainforest Research Centre Limited, Cairns (6 4pp.). Published by the Reef and Rainforest Research Centre on behalf of the Australian Government’s National Environmental Research Program (NERP) Tropical Ecosystems (TE) Hub. The Tropical Ecosystems Hub is part of the Australian Government’s National Environmental Research Program. The NERP TE Hub is administered in North Queensland by the Reef and Rainforest Research Centre Limited (RRRC). The NERP Tropical Ecosystem Hub addresses issues of concern for the management, conservation and sustainable use of the World Heritage listed Great Barrier Reef (GBR) and its catchments, tropical rainforests including the Wet Tropics World Heritage Area (WTWHA), and the terrestrial and marine assets underpinning resilient communities in the Torres Strait, through the generation and transfer of world-class research and shared knowledge. -

Introduction
Submission to the Joint Select Committee on Northern Australia James Cook University March 2014 Introduction James Cook University (JCU) welcomes the opportunity to make a submission to the Joint Select Committee on Northern Australia. JCU is Australia’s leading university in the tropics with an explicit (legislated) focus on education and research addressing the opportunities and critical challenges facing natural and human systems in the tropics, world-wide. Northern Australian is a vast natural and cultural asset of global significance. There are exciting opportunities to build on the north’s economic foundations of mining, tourism and agriculture through dynamic development and leveraging of smart technologies, place-based tropical expertise and by building economic, research and educational links with Asia Pacific and the tropical world, to develop a spectrum of products, technologies, processes and services. At the same time, though, it is important to be mindful of the fact that there are significant climatic, environmental, physical and economic constraints in northern Australia, as well as a history of overblown claims about its potential that underestimate or misrepresent these environmental, climatic and logistical realities. Universities and research institutions in the north are major employers in their own right and their operations have significant local impact. Institutions with substantial footprints and deep networks in northern Australia such as JCU have a critical role in developing the region through: Human capital -

Townsville North Queensland Tourism Opportunity Plan
Townsville North Queensland Tourism Opportunity Plan 2009–2019 DISCLAIMER – AEC GROUP LIMITED Whilst all care and diligence have been exercised in the preparation of this report, the AEC Group Limited does not warrant the accuracy of the information contained within and accepts no liability for any loss or damage that may be suffered as a result of reliance on this information, whether or not there has been any error, omission or negligence on the part of the AEC Group Limited or their employees. Any forecasts or projections used in the analysis can be affected by a number of unforeseen variables, and as such no warranty is given that a particular set of results will in fact be achieved. DISCLAIMER – STATE GOVERNMENT DISCLAIMER – TOWNSVILLE ENTERPRISE The Queensland Government makes no claim as to the accuracy of the Townsville Enterprise Ltd. makes no claim as to the accuracy of the information contained in the Townsville North Queensland Tourism information contained in the Townsville North Queensland Tourism Opportunity Plan. The document is not a prospectus and the information Opportunity Plan. The document is not a prospectus and the information provided is general in nature. The document should not be relied upon as provided is general in nature. The document should not be relied upon as the basis for financial and investment related decisions. the basis for financial and investment related decisions. Backpackers amongst Executive Summary the sugar cane, Ingham This Regional Tourism These projects are considered to represent a good cross- Opportunity Plan section of the region’s tourism values with a balance between ambition and realism for future development. -

Description of Key Species Groups in the East Marine Region
Australian Museum Description of Key Species Groups in the East Marine Region Final Report – September 2007 1 Table of Contents Acronyms........................................................................................................................................ 3 List of Images ................................................................................................................................. 4 Acknowledgements ....................................................................................................................... 5 1 Introduction............................................................................................................................ 6 2 Corals (Scleractinia)............................................................................................................ 12 3 Crustacea ............................................................................................................................. 24 4 Demersal Teleost Fish ........................................................................................................ 54 5 Echinodermata..................................................................................................................... 66 6 Marine Snakes ..................................................................................................................... 80 7 Marine Turtles...................................................................................................................... 95 8 Molluscs ............................................................................................................................