MEDICAL DRUGS PRIOR AUTHORIZATION LIST Effective 3/9/2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Minutes of the CHMP Meeting 14-17 September 2020
13 January 2021 EMA/CHMP/625456/2020 Corr.1 Human Medicines Division Committee for medicinal products for human use (CHMP) Minutes for the meeting on 14-17 September 2020 Chair: Harald Enzmann – Vice-Chair: Bruno Sepodes Disclaimers Some of the information contained in these minutes is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scopes listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also vary during the course of the review. Additional details on some of these procedures will be published in the CHMP meeting highlights once the procedures are finalised and start of referrals will also be available. Of note, these minutes are a working document primarily designed for CHMP members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the minutes cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on- going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006). 1 Addition of the list of participants Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. -

Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients
antibodies Review Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients Andrew T. Lucas 1,2,3,*, Ryan Robinson 3, Allison N. Schorzman 2, Joseph A. Piscitelli 1, Juan F. Razo 1 and William C. Zamboni 1,2,3 1 University of North Carolina (UNC), Eshelman School of Pharmacy, Chapel Hill, NC 27599, USA; [email protected] (J.A.P.); [email protected] (J.F.R.); [email protected] (W.C.Z.) 2 Division of Pharmacotherapy and Experimental Therapeutics, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] 3 Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-919-966-5242; Fax: +1-919-966-5863 Received: 30 November 2018; Accepted: 22 December 2018; Published: 1 January 2019 Abstract: The rapid advancement in the development of therapeutic proteins, including monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs), has created a novel mechanism to selectively deliver highly potent cytotoxic agents in the treatment of cancer. These agents provide numerous benefits compared to traditional small molecule drugs, though their clinical use still requires optimization. The pharmacology of mAbs/ADCs is complex and because ADCs are comprised of multiple components, individual agent characteristics and patient variables can affect their disposition. To further improve the clinical use and rational development of these agents, it is imperative to comprehend the complex mechanisms employed by antibody-based agents in traversing numerous biological barriers and how agent/patient factors affect tumor delivery, toxicities, efficacy, and ultimately, biodistribution. -

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

BLA 761125 Page 7
BLA 761125 Page 7 HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------WARNINGS AND PRECAUTIONS---------------------- These highlights do not include all the information needed to use BEOVU Endophthalmitis and retinal detachments may occur following intravitreal safely and effectively. See full prescribing information for BEOVU. injections. Patients should be instructed to report any symptoms suggestive of endophthalmitis or retinal detachment without delay (5.1). BEOVU® (brolucizumab-dbll) injection, for intravitreal injection Increases in intraocular pressure (IOP) have been seen within 30 minutes of Initial U.S. Approval: 2019 an intravitreal injection (5.2). ----------------------------INDICATIONS AND USAGE------------------------- There is a potential risk of arterial thromboembolic events (ATE) following BEOVU is a human vascular endothelial growth factor (VEGF) inhibitor intravitreal use of VEGF inhibitors (5.3). indicated for the treatment of Neovascular (Wet) Age-Related Macular ------------------------------ADVERSE REACTIONS----------------------------- Degeneration (AMD) (1). The most common adverse reactions (≥ 5%) reported in patients receiving ----------------------DOSAGE AND ADMINISTRATION---------------------- BEOVU are vision blurred (10%), cataract (7%), conjunctival hemorrhage BEOVU is administered by intravitreal injection. The recommended dose for (6%), eye pain (5%), and vitreous floaters (5%) (6.1). BEOVU is 6 mg (0.05 mL of 120 mg/mL solution) monthly (approximately To report SUSPECTED ADVERSE REACTIONS, contact Novartis every 25-31 days) for the first three doses, followed by one dose of 6 mg (0.05 Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA mL) every 8-12 weeks (2). 1088 or www.fda.gov/medwatch. ---------------------DOSAGE FORMS AND STRENGTHS-------------------- See 17 for PATIENT COUNSELING INFORMATION. Injection: 6 mg/0.05 mL solution for intravitreal injection in a single-dose vial (3). -

Refreshing the Biologic Pipeline 2020
news feature Credit: Science Lab / Alamy Stock Photo Refreshing the biologic pipeline 2020 In the absence of face-to-face meetings, FDA and industry implemented regulatory workarounds to maintain drug and biologics approvals. These could be here to stay. John Hodgson OVID-19 might have been expected since 1996) — a small miracle in itself “COVID-19 confronted us with the need to severely impair drug approvals (Fig. 1 and Table 1). to better triage sponsors’ questions,” says Cin 2020. In the event, however, To the usual crop of rare disease and Peter Marks, the director of the Center for industry and regulators delivered a small genetic-niche cancer treatments, 2020 Biologics Evaluation and Research (CBER) miracle. They found workarounds and also added a chimeric antigen receptor at the FDA. “That was perhaps the single surrogate methods of engagement. Starting (CAR)-T cell therapy with a cleaner biggest takeaway from the pandemic related in January 2020, when the outbreak veered manufacturing process and the first to product applications.” Marks says that it westward, the number of face-to face approved blockbuster indication for a became very apparent with some COVID- meetings declined rapidly; by March, small-interfering RNA (siRNA) — the 19-related files that resolving a single they were replaced by Webex and Teams. European Medicines Agency’s (EMA) issue can help a sponsor enormously and (Secure Zoom meeting are to be added registration of the RNA interference accelerate the development cycle. Before this year.) And remarkably, by 31 December, (RNAi) therapy Leqvio (inclisiran) for COVID-19, it was conceivable that a small the US Food and Drug Administration cardiovascular disease. -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Evkeeza™ (Evinacumab-Dgnb) NOTICE
DRUG POLICY Evkeeza™ (evinacumab-dgnb) NOTICE This policy contains information which is clinical in nature. The policy is not medical advice. The information in this policy is used by Wellmark to make determinations whether medical treatment is covered under the terms of a Wellmark member's health benefit plan. Physicians and other health care providers are responsible for medical advice and treatment. If you have specific health care needs, you should consult an appropriate health care professional. If you would like to request an accessible version of this document, please contact customer service at 800-524-9242. BENEFIT APPLICATION Benefit determinations are based on the applicable contract language in effect at the time the services were rendered. Exclusions, limitations or exceptions may apply. Benefits may vary based on contract, and individual member benefits must be verified. Wellmark determines medical necessity only if the benefit exists and no contract exclusions are applicable. This policy may not apply to FEP. Benefits are determined by the Federal Employee Program. DESCRIPTION The intent of the Evkeeza™ policy is to provide coverage consistent with product labeling, FDA guidance, standards of medical practice, evidence-based drug information, and/or published guidelines. The indications below including FDA-approved indications and compendial uses are considered covered benefits provided that all the approval criteria are met and the member has no exclusions to the prescribed therapy. FDA-Approved Indications Evkeeza (evinacumab-dgnb) is an ANGPTL3 (angiopoietin-like 3) inhibitor indicated as an adjunct to other low-density lipoprotein-cholesterol (LDL-C) lowering therapies for the treatment of adult and pediatric patients, aged 12 years and older, with homozygous familial hypercholesterolemia (HoFH). -
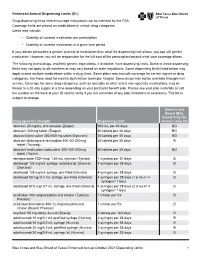
Enhanced Annual Drug List Dispensing Limits
Enhanced Annual Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral solution (Ziagen) 960 mL per 30 days BG abacavir 300 -

Regeneron Needs a New Plan B for Eylea
November 27, 2017 Regeneron needs a new plan B for Eylea Madeleine Armstrong Regeneron’s efforts to extend the lifespan of its blockbuster Eylea franchise via combinations have fallen flat again, raising the question of whether it can find anything that can best Eylea alone. With competition on the horizon from Novartis’s rival wet age-related macular degeneration (AMD) project, brolucizumab, Eylea sales are forecast to flatten after 2020 – something that Regeneron cannot now counter with combos (see table below). Regeneron’s stock was down 3% in premarket trading this morning, but the shares opened down just 1%. Investors might have been reassured by the fact that full data from the Hawk and Harrier studies of brolucizumab, released earlier this month, were not the smash hit that Novartis had hoped for. This could allow Eylea to keep its dominant position in the wet age-related macular degeneration (AMD) market for some time to come. Top wet AMD drugs in 2022 Annual indication sales ($m) Product Company Status 2016 2018e 2020e 2022e Eylea Regeneron/Bayer/Santen Pharmaceutical Marketed 3,584 3,875 4,073 3,942 Lucentis Novartis/Roche Marketed 2,354 2,271 1,924 1,460 Brolucizumab Novartis Phase III - - 319 937 Source: EvaluatePharma. And if positive, the phase III Panorama study of Eylea monotherapy in diabetic retinopathy, due to report in the first half of next year, could open up another avenue for growth for the company’s most valuable product. But even the most optimistic Regeneron bulls will have to admit that Eylea’s sales are likely to be eroded by the market entry of brolucizumab, expected in 2019 or 2020. -

July 1, 2020 RE: Physician Administered Drugs (J Codes)
July 1, 2020 RE: Physician Administered Drugs (J Codes) Included in Prior Authorization Matrix for Molina Healthcare of New York, Inc. Dear Participating Provider: Molina Healthcare of New York, Inc. requires prior authorization for these HCPCS codes for all participating providers. Prior authorization is required before services are rendered for the codes listed. Prior authorization requests should include the most up to date patient information including office notes, consultations, labs, scans, previous treatments tried, and any other patient specific information to support the request. Please fax a completed prior authorization form along with all supporting clinical documentation to fax number: 1-844- 823-5479. Our prior authorization form and most up to date formulary can be found on our website: MolinaHealthcare.com. C9061 INJECTION, TEPROTUMUMAB-TRBW, 10 mg C9063 INJECTION, EPTINEZUMAB-JJMR, 1 mg C9122 MOMETASONE FUROATE SINUS IMPLANT, 10 micrograms (sinuva) J0122 INJECTION, ERAVACYCLINE, 1 mg J0129 Injection, abatacept 10 mg J0178 Injection, aflibercept 1 mg J0179 INJECTION, BROLUCIZUMAB-DBLL, 1MG J0185 Injection, aprepitant 1 mg J0223 INJECTION, GIVOSIRAN, 0.5 mg J0285 INJECTION, AMPHOTERICIN B, 50 mg J0490 Injection, belimumab 10 mg J0517 Injection, benralizumab 1 mg J0567 Injection, cerliponase alfa 1 mg J0584 Injection, burosumab-twza 1 mg J0585 Injection, onabotulinumtoxina, 1 unit J0587 Injection, rimabotulinumtoxinb 100 units J0599 Injection, c-1 esterase inhibitor 10 units J0641 Injection, levoleucovorin, not otherwise -

Pulmonary Delivery of Biological Drugs
pharmaceutics Review Pulmonary Delivery of Biological Drugs Wanling Liang 1,*, Harry W. Pan 1 , Driton Vllasaliu 2 and Jenny K. W. Lam 1 1 Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong, China; [email protected] (H.W.P.); [email protected] (J.K.W.L.) 2 School of Cancer and Pharmaceutical Sciences, King’s College London, 150 Stamford Street, London SE1 9NH, UK; [email protected] * Correspondence: [email protected]; Tel.: +852-3917-9024 Received: 15 September 2020; Accepted: 20 October 2020; Published: 26 October 2020 Abstract: In the last decade, biological drugs have rapidly proliferated and have now become an important therapeutic modality. This is because of their high potency, high specificity and desirable safety profile. The majority of biological drugs are peptide- and protein-based therapeutics with poor oral bioavailability. They are normally administered by parenteral injection (with a very few exceptions). Pulmonary delivery is an attractive non-invasive alternative route of administration for local and systemic delivery of biologics with immense potential to treat various diseases, including diabetes, cystic fibrosis, respiratory viral infection and asthma, etc. The massive surface area and extensive vascularisation in the lungs enable rapid absorption and fast onset of action. Despite the benefits of pulmonary delivery, development of inhalable biological drug is a challenging task. There are various anatomical, physiological and immunological barriers that affect the therapeutic efficacy of inhaled formulations. This review assesses the characteristics of biological drugs and the barriers to pulmonary drug delivery.