Efficacy of Targeted Phototherapy in Idiopathic Guttate Hypomelanosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Update on Challenging Disorders of Pigmentation in Skin of Color Heather Woolery-Lloyd, M.D
Update on Challenging Disorders of Pigmentation in Skin of Color Heather Woolery-Lloyd, M.D. Director of Ethnic Skin Care Voluntary Assistant Professor Miller/University of Miami School of Medicine Department of Dermatology and Cutaneous Surgery What Determines Skin Color? What Determines Skin Color? No significant difference in the number of melanocytes between the races 2000 epidermal melanocytes/mm2 on head and forearm 1000 epidermal melanocytes/mm2 on the rest of the body differences present at birth Jimbow K, Quevedo WC, Prota G, Fitzpatrick TB (1999) Biology of melanocytes. In I. M. Freedberg, A.Z. Eisen, K. Wolff,K.F. Austen, L.A. Goldsmith, S. I. Katz, T. B. Fitzpatrick (Eds.), Dermatology in General Medicine 5th ed., pp192-220, New York, NY: McGraw Hill Melanosomes in Black and White Skin Black White Szabo G, Gerald AB, Pathak MA, Fitzpatrick TB. Nature1969;222:1081-1082 Jimbow K, Quevedo WC, Prota G, Fitzpatrick TB (1999) Biology of melanocytes. In I. M. Freedberg, A.Z. Eisen, K. Wolff, K.F. Austen, L.A. Goldsmith, S. I. Katz, T. B. Fitzpatrick (Eds.), Dermatology in General Medicine 5th ed., pp192- 220, New York, NY: McGraw Hill Role of Melanin-Advantages Melanin absorbs and scatters energy from UV and visible light to protect epidermal cells from UV damage Disadvantages Inflammation or injury to the skin is almost immediately accompanied by alteration in pigmentation Hyperpigmentation Hypopigmentation Dyschromias Post-Inflammatory hyperpigmentation Acne Melasma Lichen Planus Pigmentosus Progressive Macular Hypomelanosis -

Hydroxychloroquine-Associated Hyperpigmentation Mimicking Elder Abuse
Dermatol Ther (Heidelb) (2013) 3:203–210 DOI 10.1007/s13555-013-0032-z CASE REPORT Hydroxychloroquine-Associated Hyperpigmentation Mimicking Elder Abuse Philip R. Cohen To view enhanced content go to www.dermtherapy-open.com Received: June 17, 2013 / Published online: August 14, 2013 Ó The Author(s) 2013. This article is published with open access at Springerlink.com ABSTRACT cleared of suspected elder abuse. A skin biopsy of the patient’s dyschromia confirmed the Background: Hydroxychloroquine may result diagnosis of hydroxychloroquine-associated in cutaneous dyschromia. Older individuals hyperpigmentation. who are the victims of elder abuse can present Conclusion: Hyperpigmentation of skin, with bruising and resolving ecchymoses. mucosa, and nails can be observed in patients Purpose: The features of hydroxychloroquine- treated with antimalarials, including associated hyperpigmentation are described, hydroxychloroquine. Elder abuse is a significant the mucosal and skin manifestations of elder and underreported problem in seniors. abuse are reviewed, and the mucocutaneous Cutaneous findings can aid in the discovery of mimickers of elder abuse are summarized. physical abuse, sexual abuse, and self-neglect in Case Report: An elderly woman being treated elderly individuals. However, medication- with hydroxychloroquine for systemic lupus associated effects, systemic conditions, and erythematosus developed drug-associated black accidental external injuries can mimic elder and blue pigmentation of her skin. The abuse. Therefore, a complete medical history dyschromia was misinterpreted by her and appropriate laboratory evaluation, including clinician as elder abuse and Adult Protective skin biopsy, should be conducted when the Services was notified. The family was eventually diagnosis of elder abuse is suspected. Keywords: Abuse; Dyschromia; Elderly; P. -

Light-Based Treatment of Pigmented Lesions E
CosmetiC teChnique Light-Based Treatment of Pigmented Lesions E. Victor Ross, MD Pigmented lesions are common among patients presenting to dermatologists. Fortunately, a large arsenal of light-based technology is available for the reduction and removal of these lesions. By developing a comprehensive understanding of the microanatomy of dyschromia, physicians can optimize protocols based on principles of laser-tissue interactions and reports in the literature. This article will examine the author’s experience and preferences in treating specific types of pigmented lesions with various lasers and light devices. Cosmet Dermatol.COS 2011;24:515-522. DERM ermatologists commonly treat patients laser and nonlaser devices can be employed, sometimes with pigmented lesions. A large arsenal with different wavelengths, spot sizes, and pulse widths, of light-based devices are available for the but still achieve similar results.1 reduction and removal of these lesions. Chemical peels and cryotherapy at one time were wor- The most important factor to consider thy opponents of the laser but can present challenges Dwhen choosing the best light source for the treatment of related to damage confinement. For generalized superfi- pigmentedDo lesions is the microanatomy Not of the dyschro- cial pigmentCopy reduction, chemical peeling in experienced mia. A crucial factor in treatment of excessive pigment hands is quite sufficient. However, in focal destruction, is having an understanding of the pigment distribution the agent often will extend beyond the perimeter of the with the lesion. Lesion microanatomy should be con- lesion, even with careful application. Cryotherapy also is sidered when strategizing for optimal removal. It also notoriously challenging to control. -
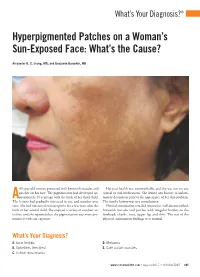
Hyperpigmented Patches on a Woman's Sun-Exposed Face
What’s Your Diagnosis?® Hyperpigmented Patches on a Woman’s Sun-Exposed Face: What’s the Cause? Alexander K. C. Leung, MD, and Benjamin Barankin, MD 45-year-old woman presented with brownish macules and Her past health was unremarkable, and she was not on any patches on her face. The pigmentation had developed ap- topical or oral medications. She denied any history of inflam- A proximately 10 years ago with the birth of her third child. matory dermatosis prior to the appearance of her skin problem. The lesions had gradually increased in size and number over The family history was not contributory. time. She had taken oral contraceptives for a few years after the Physical examination revealed symmetric, well-circumscribed, birth of her second child. She enjoyed a variety of outdoor ac- brownish macules and patches with irregular borders on the tivities, and she reported that the pigmentation was more pro- forehead, cheeks, nose, upper lip, and chin. The rest of the nounced with sun exposure. physical examination findings were normal. What’s Your Diagnosis? A. Solar lentigo D. Melasma B. Ephelides (freckles) E. Café au lait macules C. Actinic dyschromia www.consultant360.com • August 2017 • CONSULTANT 485 What’s Your Diagnosis?® Answer: Melasma A diagnosis of melasma was made. Wood lamp examination basement membrane disruption and dermal changes, indepen- showed accentuation of pigmentation suggestive of epidermal dent of UV radiation.1,17 pigmentation. The patient was treated with a series of trichlo- roacetic acid chemical peels, as well as a topical azelaic acid and HISTOPATHOLOGY a retinoid-hydroquinone-steroid (modified Kligman) formula- Three histologic patterns of pigmentation have been described: tion with significant, albeit incomplete, improvement. -

Review on Skin Hyperpigmentation: Etiology, Diagnosis and Treatment
J Pharm Sci Bioscientific Res. 2020. 10 (1):142-148 ISSN NO. 2271-3681 Review on Skin Hyperpigmentation: Etiology, Diagnosis and Treatment Sohan A. Patel*1, Jayant. B. Dave2, Timir Y. Mehta3 1. Assistant Professor, Department of Pharmacology, Smt. S M. Shah Pharmacy College, Amasaran, Mahedavad- Ahmedabad, Highway, Gujarat, India 2. Professor, Department of Quality Assurance, L. M. College of Pharmacy, Ahmedabad, Gujarat, India 3. Dermatologist, Samarpan Medical Research Organization, Modasa, Gujarat, India Article History: ABSTRACT: Received 29 April 2020 Hyperpigmentation is common dermatological condition. Skin color is determined Accepted 03 May 2020 Available online 11 May 2020 by melanin and other chromophores and is influenced by physical factors (ultraviolet radiation) and other endocrine, autocrine, and paracrine factors. Being Citation: the largest organ of the body, any abnormality in skin color can have impact on the Patel S. A., Dave J. B., Mehta T. Y., Review patients’ psychosocial impairment of life. In Indian population have verities of on Skin Hyperpigmentation: Etiology, Diagnosis and Treatment. J Pharm Sci hyperpigmentation condition on skin due to multifactorial region; hence, a Bioscientific Res. 2020. 10(1):142-148 multipronged approach is needed in such cases. The biggest challenge in such cases is to treat the hyperpigmentation itself; hence, counseling and general treatment *For Correspondence: (the use of broad-spectrum sunscreen, the avoidance of sun exposure, etc.) play Mr. Sohan A. Patel crucial role, and an interdisciplinary approach may be required, especially when Assistant professor, Department of the hyperpigmentation is due to a systemic cause. A thorough understanding of Pharmacology, Smt. S. M. Pharmacy the aetiology and management strategies of hyperpigmentation is of importance College, Amasaran, Bhumapura in caring for those afflicted and also in the development of new therapies. -

Case Report Progressive Macular Hypomelanosis: a Rarely Diagnosed Hypopigmentation in Caucasians
Hindawi Publishing Corporation Dermatology Research and Practice Volume 2009, Article ID 607682, 4 pages doi:10.1155/2009/607682 Case Report Progressive Macular Hypomelanosis: A Rarely Diagnosed Hypopigmentation in Caucasians Sven Neynaber, Christina Kirschner, Stefanie Kamann, Gerd Plewig, and Michael J. Flaig Department of Dermatology and Allergology, Ludwig-Maximilians University, Frauenlobstrasse 9-11, DE-80337 Munich, Germany Correspondence should be addressed to Sven Neynaber, [email protected] Received 4 February 2009; Accepted 24 April 2009 Recommended by Iris Zalaudek A 35-year-old woman who developed whitish macules on trunk and limbs at 12 years of age and observed a remarkable increase of the hypopigmentated lesions after her pregnancies at ages 29 and 32 years. Because of the highly characteristic clinical aspect and the light- and electron-microscopic histopathologic findings, we diagnosed progressive macular hypomelanosis (PMH). It is a nonscaly disorder with hypopigmented macules mainly on the trunk and is more often seen in young women. In contrast to some authors assuming the presence of Propionibacterium spp. as a matter of principle in PMH, we report a case with no evidence for Propionibacterium spp. Copyright © 2009 Sven Neynaber et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. 1. Introduction the nonaffected skin was remarkably intensified. She had not been on antibiotics, birth-control or hormone, containing Hypopigmented lesions of the skin are common and display pills, had no noteworthy history of dermatitis or eczema. -

Treatment of Multiple Skin Conditions with Advanced Broadband Lightbbl
Treatment of Multiple Skin Conditions with Advanced BroadBand Light BBL™ : My Experience with Asian Patients KEI NEGISHI, MD, PhD Aoyama Institute of Women’s Medicine, Tokyo Women’s Medical University; Tokyo, Japan INTRODUCTION In Asian skin, the most common and severe manifestations of photodamage are pigmentary disorders (1). Ablative resurfacing with pulsed CO2 lasers, although effective, have not found wide acceptance by Asian patients because downtime is long and hyperpigmentation, hypopigmentation, hypertrophic scars, and other post treatment complications may persist for up to one year (2). The groundbreaking flash lamp technology study by Dr. Patrick Bitter, Jr. (3) showed that a series of four to six full-face treatments with a non-ablative, non-coherent, filtered, visible light device provided improvement in all aspects of photodamage, including irregular pigmentation, skin smoothness, facial erythema, and telangiectasias. Dr. Bitter’s report was followed by a series of papers from our group describing the use of similar devices to rejuvenate Asian skin, the addition of contact cooling to reduce post-treatment complications, and the acquisition of objective spectrophotometric data to document improvements in epidermal pigmentation and skin tone in Japanese patients (4-6). An additional study describes the use of adjunctive epidermal care by hydroquinone and tretinoin cream between treatments to enhance the efficacy (7). Treatment of melasma by flash lamp on Asian skin is also reported (8); however, its use needs careful attention since melasma can easily become worse by aggressive parameters of flash lamp irradiation (9). In the author’s experience, skin attributes such as pigment and scars are more refractory in Asian patients than patients with lighter skin. -

The Stepwise Approach to the Treatment of Melasma
HIGHLIGHTING SKIN OF COLOR The Stepwise Approach to the Treatment of Melasma Maritza I. Perez, MD Melasma is a symmetric progressive hyperpig- of the pigment within the skin and the reflection of mentation of facial skin that occurs in all races light determine the perception of color. Epidermal but has a predilection for darker skin pheno- pigment is usually light to dark brown; dermal pig- types. Melasma has been associated with hor- ment is gray-blue. On Wood light examination, pig- monal imbalances, sun damage, and genetic ment that resides within the epidermis is predisposition. Clinically, melasma can be accentuated, while dermal pigment is not enhanced. divided into centrofacial, malar, and mandibular Fortunately, epidermal melasma is the most common according to the pigment distribution on the form and also the most treatable.1 Dermal melasma, skin. On Wood light examination, the pigment however, is very difficult to treat because the pig- can be found within the epidermis, where it will ment is entrapped in the dermis in the melanin enhance, or within the dermis, where it will not granules within the melanophages. To date, very few enhance. Melasma can be classified as mild, treatments are effective for dermal melasma. moderate, or severe for evaluation and treatment Clinically, facial melasma is described in accor- purposes. In this article, we will discuss the dance with the anatomic location of the discol- objective evaluation of the patient with melasma, oration.2 The centrofacial area is the most as well as the treatments based on disease commonly affected area, as seen in two thirds of severity. -

Treatment of Hyperpigmentation Heather Woolery-Lloyd, MD,*,† and Jenna N
Treatment of Hyperpigmentation Heather Woolery-Lloyd, MD,*,† and Jenna N. Kammer, BA‡ Hyperpigmentation is a common dermatologic condition that is seen in all skin types but is most prominent in skin of color. In skin of color, any inflammation or injury to skin can almost immediately be accompanied by alterations in pigmentation, either hyperpigmen- tation or hypopigmentation. Post-inflammatory hyperpigmentation can be observed in many skin conditions including acne, eczema, and contact dermatitis and treatment can be challenging. The goal is to reduce the hyperpigmentation without causing undesirable hypopigmentation or irritation in the surrounding area. This review will discuss current research on treatments for hyperpigmentation and approaches to treating this condition. Semin Cutan Med Surg 30:171-175 © 2011 Elsevier Inc. All rights reserved. KEYWORDS hyperpigmentation, hydroquinone, dyschromia, ochronosis, melasma yperpigmentation is a common dermatologic condition none is available over-the-counter in the United States and Hthat is found in all skin types but is most prominent in Canada. Four percent hydroquinone is available by prescrip- skin of color. Any inflammation or injury to the skin can tion.3 The Kligman formula combines hydroquinone with a almost immediately be accompanied by alterations in pig- retinoid and a steroid, which enhances efficacy.3 mentation, either hyperpigmentation or hypopigmentation. A common side effect when one uses hydroquinone to Postinflammatory hyperpigmentation appears in many skin treat dyschromia attributable to acne is the “hydroquinone conditions, including acne, eczema, and contact dermatitis, halo.” This occurrence is characterized by a halo of hypopig- and treatment can be challenging. The goal is to reduce the mentation surrounding the dark macule attributable to the hyperpigmentation without causing undesirable hypopig- bleaching of the surrounding normal skin. -

Cosmetic Interventions for Dyschromia: Chemical Peels Cosmetic Interventions for Dyschromia: Chemical Peels
Cosmetic Interventions for Dyschromia: Chemical Peels Cosmetic Interventions for Dyschromia: Chemical Peels Dr. Neelam A. Vashi, MD, Department of Dermatology, Boston University School of Medicine, Boston Medical Center, Boston INTRODUCTION sulfur, and limestone were used later Social psychology has long shown us by the Greeks and Romans.7In the late that attractiveness ratings of images 1800s, dermatologists pioneered skin relate positively to parameters of skin peeling for therapeutic benefit publishing homogeneity.1Faces with even skin data on the removal of ephelides with color distribution attract more visual phenol.7 Today, we define chemical attention than those with greater color peeling as the application of chemical contrast illustrating the importance exfoliating agents to the skin which of skin color homogeneity with results in destruction of one or more perceptions of beauty.2,3,4Alexis et al parts of the epidermis and/or dermis showed that dyschromia was one of the with subsequent regrowth of these 5 most common diagnoses observed layers.8 at one dermatology center amongst darkly pigmented patients.5In particular, PEELING AGENTS melasma and post-inflammatory Peeling agents can broadly be classified hyperpigmentation are major concerns into different groups such asalpha- for skin of color patients. Pigment hydroxy acids and beta-hydroxy acids. deposition in the skin secondary to Other types of peeling agents include trauma from mechanical affects and/ trichloroacetic acid, retinoic acid, or inflammatory skin disease can phenol, and combination products, cause extreme dissatisfaction amongst i.e. Jessner’s solution. The following patients and therapeutic challenges discussion will focus on superficial and to the physician. The treatment of medium peels most safe and efficacious dyschromia in darkly pigmented patients in skin types III-VI. -

Boards Fodder Disorders of Dyschromia (Hypo- and Hyperpigmentation) by Parin Pearl Rimtepathip, MD, and Janna Mieko Vassantachart, MD
boards fodder Disorders of dyschromia (hypo- and hyperpigmentation) by Parin Pearl Rimtepathip, MD, and Janna Mieko Vassantachart, MD Genetic conditions Gene Disorder Pathophysiology Clinical Features (Unique Features) Mutation Dyskeratosis XLR (MC): Reduced telomerase activ- Male > Female. Bone marrow failure up to Congenita DKC 1 ity and abnormally short- 90% (increase risk of hematopoietic malig- (Zinsser-Engman- ened telomeres chro- nancies) + triad of abnormal skin pigmenta- Cole syndrome) AD: TERT, mosomal instability/cellu- tion (poikilodermatous patches of face/neck/ TERC lar replication dysfunction upper torso), onychodystrophy, premalignant oral leukoplakia (vs benign oral leukoplakia in Pachyonychia Congenita type I) Dyschromatosis AD: ADAR Heterozygous mutations in Presents by 6-years-old with hyper/hypopig- Symmetrica (SDAR the gene encodes an RNA mented macules restricted to sun-exposed Hereditaria gene) specific adenosine deami- skin on the dorsal aspects of bilateral (Reticulate nase extremities and face Acropigmentation of Dohi) Naegeli- AD: Location of expression of Allelic to DPR. Brown gray reticulated hyper- Franceschetti- Keratin keratin 14 - Basal kerati- pigmentation typically localized to abdomen, Jadassohn 14 nocytes develops around age 2 and improves after Syndrome (NFJS) puberty. Other findings: PPK + adermato- glyphia (no finger prints) + dental anomalies including early loss of teeth (not seen in DPR) + hypohidrosis + onychodystrophy Dermatopathia AD: Location of expression of Allelic to NFJS. Unique features: diffuse non- Pigmentosa Keratin keratin 14 - Basal kerati- scarring alopecia (not seen in NFJS) + ony- Reticularis (DPR) 14 nocytes chodystrophy + adermatoglyphia + persistent reticulated hyperpigmentation of torso and proximal UE + No dental anomalies Dyschromatosis AD/AR: Mutation in ATP bind- Japanese. Torso predominant with mottled Universalis ABCB6 ing cassette subfamily B, appearance, nail dystrophy, and pterygium. -

Pediatric Dermatology in Skin of Color
Neonatal Pustular Melanosis Mongolian Spots Neonatal Pustular Melanosis TNPM is a common condition Affects 4%-5% of AA children at birth as compared to 0.1%-.3% of white infants Benign Self limited No treatment necessary Neonatal Pustular Melanosis Prominent pustules, especially on the palms and soles No erythema After rupture, peripheral collarettes of scale observed Hyperpigmentation develops, which may last for several months. Mongolian Spots Prevelance 10% white 50% Hispanic 90-100% Asian and African descent Possible association with inborn errors of metabolism Based primarily on case reports Most cases described with widespread MS GM1 gangliosidosis, Hurler’s disease, Hunter’s syndrome, mucolipidosis, Niemann-Pick disease and mannosidosis Persistence beyond one year associated with extrasacral position size larger than 10 cm dark-colored lesions (blue/blue-black) Pediatr Dermatol. 2013 Nov-Dec;30(6):683-8. doi: 10.1111/pde.12191. Epub 2013 Jul 9. Atopic Dermatitis Atopic Dermatitis More common in AA children AA race is considered a risk factor for having atopic dermatitis in the first 6 months of life Shaw TE, Currie GP, Koudelka CWet al.Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol2011;131:67–73 Moore, Megan M., et al. "Perinatal predictors of atopic dermatitis occurring in the first six months of life." Pediatrics 113.3 (2004): 468-474. Atopic Dermatitis Prevalence in pediatric population African Americans 15.9% Caucasians 9.7% Shaw TE, Currie GP, Koudelka CWet al.Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health.