Letter Granting EUA Amendment Moderna COVID-19 Vaccine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -
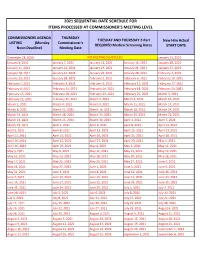
2021 Sequential Date List
2021 SEQUENTIAL DATE SCHEDULE FOR ITEMS PROCESSED AT COMMISSIONER'S MEETING LEVEL COMMISSIONERS AGENDA THURSDAY TUESDAY AND THURSDAY 2-Part New Hire Actual LISTING (Monday Commissioner's REQUIRED Medical Screening Dates START DATE Noon Deadline) Meeting Date December 28, 2020 NO MEETING SCHEDULED January 13, 2021 January 4, 2021 January 7, 2021 January 12, 2021 January 14, 2021 January 20, 2021 January 11, 2021 January 14, 2021 January 19, 2021 January 21, 2021 January 27, 2021 January 18, 2021 January 21, 2021 January 26, 2021 January 28, 2021 February 3, 2021 January 25, 2021 January 28, 2021 February 2, 2021 February 4, 2021 February 10, 2021 February 1, 2021 February 4, 2021 February 9, 2021 February 11, 2021 February 17, 2021 February 8, 2021 February 11, 2021 February 16, 2021 February 18, 2021 February 24, 2021 February 15, 2021 February 18, 2021 February 23, 2021 February 25, 2021 March 3, 2021 February 22, 2021 February 25, 2021 March 2, 2021 March 4, 2021 March 10, 2021 March 1, 2021 March 4, 2021 March 9, 2021 March 11, 2021 March 17, 2021 March 8, 2021 March 11, 2021 March 16, 2021 March 18, 2021 March 24, 2021 March 15, 2021 March 18, 2021 March 23, 2021 March 25, 2021 March 31, 2021 March 22, 2021 March 25, 2021 March 30, 2021 April 1, 2021 April 7, 2021 March 29, 2021 April 1, 2021 April 6, 2021 April 8, 2021 April 14, 2021 April 5, 2021 April 8, 2021 April 13, 2021 April 15, 2021 April 21, 2021 April 12, 2021 April 15, 2021 April 20, 2021 April 22, 2021 April 28, 2021 April 19, 2021 April 22, 2021 April 27, 2021 April -
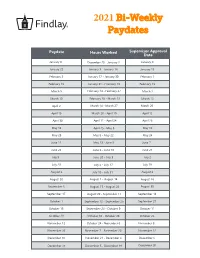
2021 Bi-Weekly Paydates
2021 Bi-Weekly Paydates Paydate Hours Worked Supervisor Approval Date January 8 December 20 - January 2 January 4 January 22 January 3 - January 16 January 15 February 5 January 17 - January 30 February 1 February 19 January 31 - February 13 February 15 March 5 February 14 - February 27 March 1 March 19 February 28 - March 13 March 15 April 2 March 14 - March 27 March 29 April 16 March 28 - April 10 April 12 April 30 April 11 - April 24 April 26 May 14 April 25 - May 8 May 10 May 28 May 9 - May 22 May 24 June 11 May 23 - June 5 June 7 June 25 June 6 - June 19 June 21 July 9 June 20 - July 3 July 2 July 23 July 4 - July 17 July 19 August 6 July 18 - July 31 August 2 August 20 August 1 - August 14 August 16 September 3 August 15 - August 28 August 30 September 17 August 29 - September 11 September 13 October 1 September 12 - September 25 September 27 October 15 September 26 - October 9 October 11 October 29 October 10 - October 23 October 25 November 12 October 24 - November 6 November 8 November 26 November 7 - November 20 November 22 December 10 November 21 - December 4 December 6 December 24 December 5 - December 18 December 20 2021 Bi-Weekly Paydate/Holiday Paydates January February March April Sun Mon Tues Wed Thurs Fri Sat Sun Mon Tues Wed Thurs Fri Sat Sun Mon Tues Wed Thurs Fri Sat Sun Mon Tues Wed Thurs Fri Sat 1 2 1 2 3 4 5 6 1 2 3 4 5 6 1 2 3 4 3 5 6 7 8 9 7 8 9 10 11 12 13 7 8 9 10 11 12 13 4 5 6 7 8 9 10 10 11 12 13 14 15 16 14 15 16 17 18 19 20 14 15 16 17 18 19 20 11 12 13 14 15 16 17 17 18 19 20 21 22 23 21 22 23 24 25 26 -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -

PSH Weekly Update
August 30, 2021 PSH Families, It appears that we will get some relief from the hot weather this week, which will allow our students’ classes to begin to settle into their routines and structures that will be in place throughout the school year. I really appreciated both our students and teachers willingness to work through the hot afternoons on both Thursday and Friday next week. A large focus of our classes this year will be for our students to engage in more frequent and focused reading, writing and discussing. With the start of the year behind us and less disruptions due to the heat, our teachers should be able to begin to establish this clear academic focus this week. Week of August 30 - News and Information PVLA - Materials Pick-up - Tuesday, August 31, 3:00pm - 5:00pm Attention PVLA students. Administration will be available from 3:00 - 5:00pm tomorrow for any PVLA students who have been told by their teachers to come and pick-up materials. Pick up will occur in the auditorium lobby. Please speak with your PVLA teacher about whether you need to pick up any materials. Dismissal: The school day ends at 2:30pm. We ask our students who are not involved in a supervised activity to be out of the building by 3:00pm rain or shine. This is a safety and supervision concern that I would appreciate your support in asking your child to make their way home in as best a way possible. Counselors and Information: Our School Counselors are now in the office to serve students and parents. -

Office of the Mayor
OFFICE OF THE MAYOR CITY AND COUNTY OF HONOLULU 530 SOUTH KING STREET, ROOM 300 • HONOLULU, HAWAII 96813 PHONE: (808) 768-4141 • FAX: (808) 768-4242 • INTERNET: www.honolulu.gov RICK BLANGIARDI MICHAEL D. FORMBY MAYOR MANAGING DIRECTOR DANETTE MARUYAMA DEPUTY MANAGING DIRECTOR August 30, 2021 OFFICE OF THE MAYOR CITY AND COUNTY OF HONOLULU EMERGENCY ORDER NO. 2021-11 (COVID-19 [Novel Coronavirus]) SECOND AMENDMENT TO THE ORDER IMPLEMENTING TIER 5 OF HONOLULU’S POST COVID-19 VACCINE FRAMEWORK By the authority vested in me as Mayor of the City and County of Honolulu (the “City”) pursuant to Revised Charter of the City and County of Honolulu 1973 (2017 Edition), as amended; the Revised Ordinances of the City and County of Honolulu 1990, as amended; the Hawai‘i Revised Statutes, as amended (“Haw. Rev. Stat.”), the Constitution and laws of the State of Hawai‘i, I, RICK BLANGIARDI, hereby issue this Second Amendment to the Order Implementing Tier 5 of Honolulu’s Post COVID-19 Vaccine Framework (“Second Amendment”) to further address the emergency initially declared via proclamation on March 4, 2020, and the subsequent Fifteenth Proclamation of Emergency or Disaster (COVID-19 [Novel Coronavirus]). I. FURTHER AMENDMENTS TO THE ORDER A. Addition of “Order 10” and “Order 11” Emergency Order No. 2021-09 (COVID-19 [Novel Coronavirus]) Order Implementing Tier 5 of Honolulu’s Post Covid-19 Vaccine Framework (the “Order”) is further amended to include “Order 10” and “Order 11” (following Order 9 on page 6 of the Order), as set forth below: Order 10: A. Effective September 13, 2021, all covered entities shall not permit a patron to enter covered premises without displaying proof of full vaccination, and identification bearing the same identifying information as the proof of full vaccination. -

2021 Working Day Calendar-5 Day-Alternative Format
2021 Working Days Calendar – 5 day The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 Non-working Day Saturday, January 2, 2021 Non-working Day Sunday, January 3, 2021 Non-working Day Monday, January 4, 2021 938 Tuesday, January 5, 2021 939 Wednesday, January 6, 2021 940 Thursday, January 7, 2021 941 Friday, January 8, 2021 942 Saturday, January 9, 2021 Non-working Day Sunday, January 10, 2021 Non-working Day Monday, January 11, 2021 943 Tuesday, January 12, 2021 944 Wednesday, January 13, 2021 945 Thursday, January 14, 2021 946 Friday, January 15, 2021 947 Saturday, January 16, 2021 Non-working Day Sunday, January 17, 2021 Non-working Day Monday, January 18, 2021 Non-working Day Tuesday, January 19, 2021 948 Wednesday, January 20, 2021 949 Thursday, January 21, 2021 950 Friday, January 22, 2021 951 Saturday, January 23, 2021 Non-working Day Sunday, January 24, 2021 Non-working Day Monday, January 25, 2021 952 Tuesday, January 26, 2021 953 Wednesday, January 27, 2021 954 Thursday, January 28, 2021 955 Friday, January 29, 2021 956 Saturday, January 30, 2021 Non-working Day Sunday, January 31, 2021 Non-working Day Monday, February 1, 2021 957 Tuesday, February 2, 2021 958 Wednesday, February 3, -

Bulletin Publication Schedule for Calendar Year 2020
BULLETIN PUBLICATION SCHEDULE FOR CALENDAR YEAR 2020 Vol. Monthly Issue ARRF Due Closing Date for 21-day Comment Publication Date No. of Bulletin to DFM Agency Filing Period End Date 20-1 January 2020 November 15, 2019 *November 29, 2019 January 1, 2020 January 22, 2020 20-2 February 2020 December 20, 2019 January 3, 2020 February 5, 2020 February 26, 2020 20-3 March 2020 January 22, 2020 February 7, 2020 March 4, 2020 March 25, 2020 20-4 April 2020 February 21, 2020 March 6, 2020 April 1, 2020 April 22, 2020 20-5 May 2020 March 20, 2020 April 3, 2020 May 6, 2020 May 27, 2020 20-6 June 2020 April 24, 2020 May 1, 2020 June 3, 2020 June 24, 2020 20-7 July 2020 May 22, 2020 June 5, 2020 July 1, 2020 July 22, 2020 20-8 August 2020 June 19, 2020 July 3, 2020 August 5, 2020 August 26, 2020 20-9 September 2020 July 24, 2020 August 7, 2020 September 2, 2020 September 23, 2020 20-10 October 2020 August 21, 2020 **August 28, 2020 October 7, 2020 October 28, 2020 20-11 November 2020 September 18, 2020 October 2, 2020 November 4, 2020 November 25, 2020 20-12 December 2020 October 23, 2020 November 6, 2020 December 2, 2020 December 23, 2020 BULLETIN PUBLICATION SCHEDULE FOR CALENDAR YEAR 2021 Vol. Monthly Issue ARRF Due Closing Date for Bulletin 21-day Comment No. of Bulletin to DFM Agency Filing Publication Date Period End Date 21-1 January 2021 November 16, 2021 *November 30, 2020 January 6, 2021 January 27, 2021 21-2 February 2021 December 24, 2021 January 8, 2021 February 3, 2021 February 24, 2021 21-3 March 2021 January 22, 2021 February 5, 2021 -

Utility Billing Dates 2021
2021 Reading & Billing Schedule for Website.xlsx Billing Month Cycle Start Read date Finish read date Billing Date Due Date & Bank Draft Date Penalty Date Pay by dates to avoid cut off Cut off date January 1 Monday, December 21, 2020 Thursday, December 24, 2020 Thursday, January 7, 2021 Thursday, January 28, 2021 Tuesday, February 2, 2021 Tuesday, February 16, 2021 Wednesday, February 17, 2021 2 Monday, December 28, 2020 Thursday, December 31, 2020 Thursday, January 14, 2021 Thursday, February 4, 2021 Tuesday, February 9, 2021 Tuesday, February 23, 2021 Wednesday, February 24, 2021 3 Monday, January 4, 2021 Thursday, January 7, 2021 Thursday, January 21, 2021 Thursday, February 11, 2021 Tuesday, February 16, 2021 Tuesday, March 2, 2021 Wednesday, March 3, 2021 4 Monday, January 11, 2021 Thursday, January 14, 2021 Thursday, January 28, 2021 Thursday, February 18, 2021 Tuesday, February 23, 2021 Tuesday, March 9, 2021 Wednesday, March 10, 2021 February 1 Tuesday, January 19, 2021 Friday, January 22, 2021 Thursday, February 4, 2021 Thursday, February 25, 2021 Tuesday, March 2, 2021 Tuesday, March 16, 2021 Wednesday, March 17, 2021 2 Monday, January 25, 2021 Thursday, January 28, 2021 Thursday, February 11, 2021 Thursday, March 4, 2021 Tuesday, March 9, 2021 Tuesday, March 23, 2021 Wednesday, March 24, 2021 3 Monday, February 1, 2021 Thursday, February 4, 2021 Thursday, February 18, 2021 Thursday, March 11, 2021 Tuesday, March 16, 2021 Tuesday, March 30, 2021 Wednesday, March 31, 2021 4 Monday, February 8, 2021 Thursday, February 11, 2021