Cervical Cancer View Online At
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Curative Pelvic Exenteration for Recurrent Cervical Carcinoma in the Era of Concurrent Chemotherapy and Radiation Therapy
Available online at www.sciencedirect.com ScienceDirect EJSO xx (2015) 1e11 www.ejso.com Review Curative pelvic exenteration for recurrent cervical carcinoma in the era of concurrent chemotherapy and radiation therapy. A systematic review H. Sardain a,b, V. Lavoue a,b,c,*, M. Redpath d, N. Bertheuil b,e, F. Foucher a,J.Lev^eque a,b,c a CHU de Rennes, Gynecology Department, Tertiary Surgery Center, Teaching Hospital of Rennes, Hopital^ Sud, 16, Bd de Bulgarie, 35000 Rennes, France b Universite de Rennes, Faculty of Medicine, 2 Henry Guilloux, 35000 Rennes, France c INSERM, ER440, Oncogenesis, Stress and Signaling (OSS), Rennes, France d McGill University, Department of Pathology, Jewish General Hospital, Cote^ Sainte Catherine, Montreal, QC, Canada e CHU de Rennes, Department of Plastic, Reconstructive and Aesthetic Surgery, Tertiary Surgery Center, Teaching Hospital of Rennes, Hopital^ Sud, 16, Bd de Bulgarie, 35000 Rennes, France Accepted 26 March 2015 Available online --- Abstract Objective: Pelvic exenteration requires complete resection of the tumor with negative margins to be considered a curative surgery. The pur- pose of this review is to assess the optimal preoperative evaluation and surgical approach in patients with recurrent cervical cancer to in- crease the chances of achieving a curative surgery with decreased morbidity and mortality in the era of concurrent chemoradiotherapy. Methods: Review of English publications pertaining to cervical cancer within the last 25 years were included using PubMed and Cochrane Library searches. Results: Modern imaging (MRI and PET-CT) does not accurately identify local extension of microscopic disease and is inadequate for pre- operative planning of extent of resection. -

Developmental Abnormalities Easy to Misdiagnose
8 Gynecology O B .GYN. NEWS • March 15, 2005 Developmental Abnormalities Easy to Misdiagnose BY JANE SALODOF MACNEIL inappropriate surgery, according to Dr. even an entity called obstructed hemi- Abnormalities of the vulva include con- Contributing Writer Zurawin, chief of the section of pediatric vagina.” genital labial fusion, which he said could and adolescent gynecology at Baylor and Clitoral hypertrophy is the only devel- be corrected with a simple flap proce- H OUSTON — Developmental abnor- of the gynecology service at Texas Chil- opmental abnormality of the clitoris, ac- dure. Surgery is rarely used, however, for malities of the vulva and vagina are often dren’s Hospital in Houston. cording to Dr. Zurawin. It used to be acquired labial agglutination. “This is one easy to correct, but also easy to misdiag- “You need to be familiar with the syn- treated by clitoridectomy with “very un- of most common referrals from pediatri- nose, Robert K. Zurawin, M.D., warned at dromes before you treat. Many people satisfactory results,” he said, describing cians, because they don’t know what to do a conference on vulvovaginal diseases are confronted with these conditions, and more conservative procedures in use to- with it and they are afraid,” Dr. Zurawin sponsored by Baylor College of Medicine. they don’t know what they really are,” he day. “This is mainly a cosmetic problem said. Many physicians have not been trained said. “With the obstructions, for example, for patients, and the surgical management He attributed most cases to diaper rash, to recognize these rare disorders and, as a they may just think it is an imperforate hy- is resection of the enlarged clitoris,” he bubble baths, and detergents that can in- result, run the risk of doing excessive or men and are not even aware that there is said. -

Pelvic Exenteration for the Management of Pelvic Malignancies
Chapter 7 Pelvic Exenteration for the Management of Pelvic Malignancies Daniel Paramythiotis, Konstantinia Kofina and Antonios Michalopoulos Additional information is available at the end of the chapter http://dx.doi.org/10.5772/61083 Abstract Pelvic exenteration is a surgical procedure first described by Brunschwig in 1948 as a curative or palliative treatment for pelvic and perineal tumors. It is actually a radical operation, involving en bloc resection of pelvic organs, including reproductive structures, bladder, and rectosigmoid. In patients with recurrent cervical and vaginal malignancy, it is associated with a 5-year survival of more than 50%. In spite of advances in surgical management, consequences such as stomas, are still frequently unavoidable for radical tumor excision. Most candidates for this procedure have been diagnosed with recurrent cervical cancer that has previously been treated with surgery and radiation, or radiation alone. Complications of pelvic exenteration are more severe than those of standard resection of a colorectal carcinoma, so it is not commonly performed, including wound infection, wound dehiscence (also described as burst abdomen) the creation of fistulae (perineal-fecal, uretero-vaginal, between conduit and perineal wound), urinary tract infections, perineal hernias and intestinal obstruction. Patients need to be carefully selected and counseled about risks and long-term issues related to the surgery. A comprehensive evaluation is required in order to exclude unresectable or metastatic disease. Evolution of the technique through laparoscopy and minimally invasive surgery may result in a reduction of morbidity and mortality. Keywords: Pelvic exenteration, gynecologic cancer 1. Introduction Pelvic exenteration was first described by Brunschwig and his colleagues of New York’s Memorial Hospital in 1948 [1] and was initially performed as a palliative surgical intervention © 2015 The Author(s). -

2021 – the Following CPT Codes Are Approved for Billing Through Women’S Way
WHAT’S COVERED – 2021 Women’s Way CPT Code Medicare Part B Rate List Effective January 1, 2021 For questions, call the Women’s Way State Office 800-280-5512 or 701-328-2389 • CPT codes that are specifically not covered are 77061, 77062 and 87623 • Reimbursement for treatment services is not allowed. (See note on page 8). • CPT code 99201 has been removed from What’s Covered List • New CPT codes are in bold font. 2021 – The following CPT codes are approved for billing through Women’s Way. Description of Services CPT $ Rate Office Visits New patient; medically appropriate history/exam; straightforward decision making; 15-29 minutes 99202 72.19 New patient; medically appropriate history/exam; low level decision making; 30-44 minutes 99203 110.77 New patient; medically appropriate history/exam; moderate level decision making; 45-59 minutes 99204 165.36 New patient; medically appropriate history/exam; high level decision making; 60-74 minutes. 99205 218.21 Established patient; evaluation and management, may not require presence of physician; 99211 22.83 presenting problems are minimal Established patient; medically appropriate history/exam, straightforward decision making; 10-19 99212 55.88 minutes Established patient; medically appropriate history/exam, low level decision making; 20-29 minutes 99213 90.48 Established patient; medically appropriate history/exam, moderate level decision making; 30-39 99214 128.42 minutes Established patient; comprehensive history exam, high complex decision making; 40-54 minutes 99215 128.42 Initial comprehensive -

Treating Cervical Cancer If You've Been Diagnosed with Cervical Cancer, Your Cancer Care Team Will Talk with You About Treatment Options
cancer.org | 1.800.227.2345 Treating Cervical Cancer If you've been diagnosed with cervical cancer, your cancer care team will talk with you about treatment options. In choosing your treatment plan, you and your cancer care team will also take into account your age, your overall health, and your personal preferences. How is cervical cancer treated? Common types of treatments for cervical cancer include: ● Surgery for Cervical Cancer ● Radiation Therapy for Cervical Cancer ● Chemotherapy for Cervical Cancer ● Targeted Therapy for Cervical Cancer ● Immunotherapy for Cervical Cancer Common treatment approaches Depending on the type and stage of your cancer, you may need more than one type of treatment. For the earliest stages of cervical cancer, either surgery or radiation combined with chemo may be used. For later stages, radiation combined with chemo is usually the main treatment. Chemo (by itself) is often used to treat advanced cervical cancer. ● Treatment Options for Cervical Cancer, by Stage Who treats cervical cancer? Doctors on your cancer treatment team may include: 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 ● A gynecologist: a doctor who treats diseases of the female reproductive system ● A gynecologic oncologist: a doctor who specializes in cancers of the female reproductive system who can perform surgery and prescribe chemotherapy and other medicines ● A radiation oncologist: a doctor who uses radiation to treat cancer ● A medical oncologist: a doctor who uses chemotherapy and other medicines to treat cancer Many other specialists may be involved in your care as well, including nurse practitioners, nurses, psychologists, social workers, rehabilitation specialists, and other health professionals. -

Colposcopy.Pdf
CCololppooscoscoppyy ► Chris DeSimone, M.D. ► Gynecologic Oncology ► Images from Colposcopy Cervical Pathology, 3rd Ed., 1998 HistoHistorryy ► ColColpposcopyoscopy wwasas ppiioneeredoneered inin GGeermrmaanyny bbyy DrDr.. HinselmannHinselmann dduriurinngg tthhee 19201920’s’s ► HeHe sousougghtht ttoo prprooveve ththaatt micmicrroscopicoscopic eexaminxaminaationtion ofof thethe cervixcervix wouwoulldd detectdetect cervicalcervical ccancanceerr eeararlliierer tthhaann 44 ccmm ► HisHis workwork identidentiifiefiedd severalseveral atatyypicalpical appeappeararanancceses whwhicichh araree stistillll usedused ttooddaay:y: . Luekoplakia . Punctation . Felderung (mosaicism) Colposcopy Cervical Pathology 3rd Ed. 1998 HistoHistorryy ► ThrThrooughugh thethe 3030’s’s aanndd 4040’s’s brbreaeaktkthrhrouougghshs wwereere mamaddee regregaarrddinging whwhicichh aapppepeararancanceess wweerere moremore liklikelelyy toto prprogogressress toto invinvaasivesive ccaarcinomrcinomaa;; HHOOWEWEVVERER,, ► TheThessee ffiinndingsdings wweerere didifffficiculultt toto inteinterrpretpret sincesince theythey werweree notnot corcorrrelatedelated wwithith histologhistologyy ► OneOne resreseaearcrchherer wwouldould claclaiimm hhiiss ppatatientsients wwithith XX ffindindiingsngs nevernever hahadd ccaarcinomarcinoma whwhililee aannothotheerr emphemphaatiticcallyally belibelieevedved itit diddid ► WorldWorld wiwidede colposcopycolposcopy waswas uunnderderuutitillizizeedd asas aa diadiaggnosticnostic tooltool sseeconcondadaryry ttoo tthheseese discrepadiscrepannciescies HistoHistorryy -

Diagnostic Tests for Vaginosis/Vaginitis
Alberta Heritage Foundation for Medical Research Diagnostic tests for vaginosis/vaginitis Christa Harstall and Paula Corabian October 1998 HTA12 Diagnostic tests for vaginosis/vaginitis Christa Harstall and Paula Corabian October 1998 © Copyight Alberta Heritage Foundation for Medical Research, 1998 This Health Technology Assessment Report has been prepared on the basis of available information of which the Foundation is aware from public literature and expert opinion, and attempts to be current to the date of publication. The report has been externally reviewed. Additional information and comments relative to the Report are welcome, and should be sent to: Director, Health Technology Assessment Alberta Heritage Foundation for Medical Research 3125 Manulife Place, 10180 - 101 Street Edmonton Alberta T5J 3S4 CANADA Tel: 403-423-5727, Fax: 403-429-3509 This study is based, in part, on data provided by Alberta Health. The interpretation of the data in the report is that of the authors and does not necessarily represent the views of the Government of Alberta. ISBN 1-896956-15-7 Alberta's health technology assessment program has been established under the Health Research Collaboration Agreement between the Alberta Heritage Foundation for Medical Research and the Alberta Health Ministry. Acknowledgements The Alberta Heritage Foundation for Medical Research is most grateful to the following persons for their comments on the draft report and for provision of information. The views expressed in the final report are those of the Foundation. Dr. Jane Ballantine, Section of General Practice, Calgary Dr. Deirdre L. Church, Microbiology, Calgary Laboratory Services, Calgary Dr. Nestor N. Demianczuk, Royal Alexandra Hospital, Edmonton Dr. -

Key Points: • a Pap Test and Pelvic Exam Are Important Parts of A
Key Points: • A Pap test and pelvic exam are important parts of a woman’s routine health care because they can detect cancer or abnormalities that may lead to cancer of the cervix (see Question 3). • Women should have a Pap test at least once every three years, beginning about three years after they begin to have sexual intercourse, but no later than age 21 (see Question 6). • If the Pap test shows abnormalities, further tests an/or treatment may be necessary (see Question 11). • Human papillomavirus (HPV) infection is the primary risk factor for cervical cancer (see Question 13). 1. What is a Pap test? The Pap test (sometimes called a Pap smear) is a way to examine cells collected from the cervix (the lower, narrow end of the uterus). The main purpose of the Pap test is to find abnormal cell changes that may arise from cervical cancer or before cancer develops. 2. What is a pelvic exam? In a pelvic exam, the uterus, vagina, ovaries, fallopian tubes, bladder, and rectum are felt to find any abnormality in their shape or size. During a pelvic exam, an instrument called a speculum is used to widen the vagina so that the upper portion of the vagina and the cervix can be seen. 3. Why are a Pap test and pelvic exam important? A Pap test and pelvic exam are important parts of a woman’s routine health care because they can detect abnormalities that may lead to invasive cancer of the cervix. These abnormalities can be treated before cancer develops. -
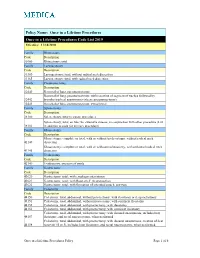
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

The Relationship Between Female Genital Aesthetic Perceptions and Gynecological Care
Examining the Vulva: The Relationship between Female Genital Aesthetic Perceptions and Gynecological Care By Vanessa R. Schick B.A. May 2004, University of Massachusetts, Amherst A Dissertation Submitted to The Faculty of Columbian College of Arts and Sciences of The George Washington University in Partial Satisfaction of the Requirements for the Degree of Doctor of Philosophy January 31, 2010 Dissertation directed by Alyssa N. Zucker Associate Professor of Psychology and Women’s Studies The Columbian College of Arts and Sciences of The George Washington University certifies that Vanessa R. Schick has passed the Final Examination for the degree of Doctor of Philosophy as of August 19, 2009. This is the final and approved form of the dissertation. Examining the Vulva: The Relationship between Female Genital Aesthetic Perceptions and Gynecological Care Vanessa R. Schick Dissertation Research Committee: Alyssa N. Zucker, Associate Professor of Psychology & Women's Studies, Dissertation Director Laina Bay-Cheng, Assistant Professor of Social Work, University at Buffalo, Committee Member Maria-Cecilia Zea, Professor of Psychology, Committee Member ii © Copyright 2009 by Vanessa R. Schick All rights reserved iii Acknowledgments The past five years have changed me and my research path in ways that I could have never imagined. I feel incredibly fortunate for my mentors, colleagues, friends and family who have supported me throughout this journey. First, I would like to start by expressing my sincere appreciation to my phenomenal dissertation committee and all those who made this dissertation possible: Without Alyssa Zucker, my advisor, my journey would have been an entirely different one. Few advisors would allow their students to forge their own research path. -

Pelvic Exenteration As Ultimate Ratio for Gynecologic Cancers: Single‑Center Analyses of 37 Cases
Archives of Gynecology and Obstetrics (2019) 300:161–168 https://doi.org/10.1007/s00404-019-05154-4 GYNECOLOGIC ONCOLOGY Pelvic exenteration as ultimate ratio for gynecologic cancers: single‑center analyses of 37 cases N. de Gregorio1 · A. de Gregorio1 · F. Ebner2 · T. W. P. Friedl1 · J. Huober1 · R. Hefty3 · M. Wittau4 · W. Janni1 · P. Widschwendter1 Received: 5 February 2019 / Accepted: 5 April 2019 / Published online: 22 April 2019 © Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Background Pelvic exenterations are a last resort procedure for advanced gynecologic malignancies with elevated risks in terms of patients’ morbidity. Methods This single-center analysis reports surgical details, outcome and survival of all patients treated with exenteration for non-ovarian gynecologic malignancies at our university hospital during a 13-year time period. We collected data regarding patients and tumor characteristics, surgical procedures, peri- and postoperative management, transfusions, complications, and analyzed the impact on survival outcomes. Results We identifed 37 patients between 2005 and 2013 with primary or relapsed cervical cancer (59.5%), vulvar cancer (24.3%) or endometrial cancer (16.2%). Median age was 60 years and most patients (73%) had squamous cell carcinomas. Median progression-free survival was 26.2 months and median overall survival was 49.9 months. The 5-year survival rates were 34.4% for progression-free survival and 46.4% for overall survival. There were no signifcant diferences in progres- sion-free survival and overall survival with regard to disease entity. Patients with tumor at the resection margins (R1) had a nearly signifcantly worse progression-free survival (median: 28.5 vs. -

Seer Program Code Manual
THE SEER PROGRAM CODE MANUAL Revised Edition June 1992 CANCER STATISTICS BRANCH SURVEILLANCE PROGRAM DIVISION OF CANCER PREVENTION AND CONTROL NATIONAL CANCER INSTITUTE U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES PUBLIC HEALTH SERVICE NATIONAL INSTITUTES OF HEALTH Effective Date: Cases Diagnosed January 1, 1992 The SEER Program Code Manual Revised Edition June 1992 Editors Jack Cunningham Lynn Ries Benjamin Hankey Jennifer Seiffert Barbara Lyles Evelyn Shambaugh Constance Percy Valerie Van Holten Acknowledgements The editors wish to acknowledge the assistance of Terry Swenson, Maureen Troublefield, Diane Licitra, and Jerome Felix of Information Management Services, Inc., in the preparation of the SEER Program Code Manual. The editors also wish to acknowledge the assistance of Dr. John Berg in preparation of the section on multiple primary determination for lymphatic and hematopoietic diseases. TABLE OF CONTENTS PREFACE TO THE REVISED EDITION ....................................... vii COMPUTER RECORD FORMAT ............................................ 1 INTRODUCTION AND GENERAL INSTRUCTIONS .............................. 5 REFERENCES .......................................................... 37 SEER CODE SUMMARY .................................................. 39 I BASIC RECORD IDENTIFICATION ...................................... 57 1.01 SEER Participant ........................................... 58 1.02 Case Number .............................................. 59 1.03 Record Number ............................................ 60