Therapeutic Polyanhydride Compounds for Drug Delivery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplement Ii to the Japanese Pharmacopoeia Fifteenth Edition
SUPPLEMENT II TO THE JAPANESE PHARMACOPOEIA FIFTEENTH EDITION Official From October 1, 2009 English Version THE MINISTRY OF HEALTH, LABOUR AND WELFARE Notice: This English Version of the Japanese Pharmacopoeia is published for the conven- ience of users unfamiliar with the Japanese language. When and if any discrepancy arises between the Japanese original and its English translation, the former is authentic. The Ministry of Health, Labour and Welfare Ministerial Notification No. 425 Pursuant to Paragraph 1, Article 41 of the Pharmaceutical Affairs Law (Law No. 145, 1960), we hereby revise a part of the Japanese Pharmacopoeia (Ministerial Notification No. 285, 2006) as follows*, and the revised Japanese Pharmacopoeia shall come into ef- fect on October 1, 2009. However, in the case of drugs which are listed in the Japanese Pharmacopoeia (hereinafter referred to as “previous Pharmacopoeia”) [limited to those listed in the Japanese Pharmacopoeia whose standards are changed in accordance with this notification (hereinafter referred to as “new Pharmacopoeia”)] and drugs which have been approved as of October 1, 2009 as prescribed under Paragraph 1, Article 14 of the same law [including drugs the Minister of Health, Labour and Welfare specifies (the Ministry of Health and Welfare Ministerial Notification No. 104, 1994) as those ex- empted from marketing approval pursuant to Paragraph 1, Article 14 of the Pharmaceu- tical Affairs Law (hereinafter referred to as “drugs exempted from approval”)], the Name and Standards established in the previous Pharmacopoeia (limited to part of the Name and Standards for the drugs concerned) may be accepted to conform to the Name and Standards established in the new Pharmacopoeia before and on March 31, 2011. -
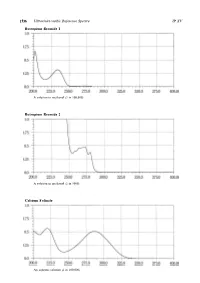
Ultraviolet-Visible Reference Spectra C
1536 Ultraviolet-visible Reference Spectra JP XV Butropium Bromide 1 A solution in methanol (1 in 100,000) Butropium Bromide 2 A solution in methanol (1 in 5000) Calcium Folinate An aqueous solution (1 in 100,000) JP XV Ultraviolet-visible Reference Spectra 1537 Camostat Mesilate An aqueous solution (1 in 100,000) Carbamazepine The sample solution obtained in the Assay Carbazochrome Sodium Sulfonate Hydrate An aqueous solution (1 in 100,000) 1538 Ultraviolet-visible Reference Spectra JP XV Carbidopa Hydrate A solution prepared as follows: Dissolve 0.01 g in 250 mL of a solution of hydrochloric acid in methanol (9 in 1000) Carmofur A solution in a mixture of methanol and phosphoric acid-acetic acid-boric acid buffer solution, pH 2.0 (9:1) (1 in 100,000) Carteolol Hydrochloride An aqueous solution (1 in 100,000) JP XV Ultraviolet-visible Reference Spectra 1539 Carumonam Sodium An aqueous solution (3 in 100,000) Cefaclor An aqueous solution (1 in 50,000) Cefadroxil An aqueous solution (1 in 50,000) 1540 Ultraviolet-visible Reference Spectra JP XV Cefalexin An aqueous solution (3 in 100,000) Cefalotin Sodium An aqueous solution (1 in 50,000) Cefapirin Sodium An aqueous solution (3 in 200,000) JP XV Ultraviolet-visible Reference Spectra 1541 Cefatrizine Propylene Glycolate An aqueous solution (1 in 50,000) Cefazolin Sodium An aqueous solution (1 in 50,000) Cefbuperazone Sodium An aqueous solution (1 in 50,000) 1542 Ultraviolet-visible Reference Spectra JP XV Cefditoren Pivoxil A solution in methanol (1 in 50,000) Cefixime A solution in 0.1 -

00246-2019.Supptables
1 Table S1. List of ICD-10 codes identifying comorbidities during hospitalization for COPD exacerbation COPD J41, J42, J43, J440, J441, J449 COPD exacerbation J10, J11, J13, J14, J15, J16, J18, J20, J21, J22, J46, J170, J171, J178, J440, J441, J851, J12, A481, B012, B052, B250 Bacterial pneumonia J100, J110, J12, J13, J14, J15, J16, J170, J178, J18, J851, A481 Lung cancer C34 Other malignancy C00-26, C30-33, C37-41, C43-58, C60-76 Diabetes/abnormal glucose tolerance E10-14, R703 Bone fracture/osteoporosis M80-84 Interstitial pneumonia B221, J701, J704, J841, J848, J849, J990, J991, M321, M330, M331, M332, M351 Bronchial asthma J45, J46 Bronchiectasis J40, J41, J42, J47 Pulmonary thromboembolism I26 Mycobacterium infection A150-154, A156-159, A161-162, A165, A168-169, A19, A310, A319 Mycotic infection A420, A43, B37, B380-382, B390-392, B400-402, B410, B420, B440, B441, B449, B460, J172 Cor pulmonare I27 Congestive heart failure E059, I46, I50, I099, I110 Ischemic heart disease I20-25 Tachycardia I47-49, R000, T818 Autoimmune disease M05, M06, M08, M30-35 Stroke I60-64 Liver dysfunction B89, B181, B182, B659, B661, K702, K703, K72, K74, K761, K762, K763, K766, K767 Renal failure E102, E112, E142, I120, N17-19 GERD K21 Constipation or ileus K56, K590 Prostate hypertrophy N40 Abbreviations: COPD, chronic obstructive pulmonary disease; GERD, gastro-esophageal reflux disease 2 Table S2. List of baseline characteristics, comorbidities, and treatments before and during hospitalization for COPD exacerbation Baseline characteristics Sex, fiscal -

Breeding Laboratories (Hino, Japan)
VOL. XLII NO. 6 THE JOURNAL OF ANTIBIOTICS 989 STIMULATORY EFFECT OF CEFODIZIME ON MACROPHAGE- MEDIATED PHAGOCYTOSIS Kazunori Oishi, Keizo Matsumoto, Masashi Yamamoto, Toshihiro Morito and Toshiaki Yoshida Department of Internal Medicine, Institute of Tropical Medicine, Nagasaki University, 12-4 Sakamoto-machi, Nagasaki 852, Japan (Received for publication January 27, 1989) Weevaluated the ingestion of anti-sheep erythrocyte (anti-E) IgG- and IgM-coated sheep erythiocytes by murineperitoneal macrophagesexposed to cefodizime, a newsemisynthetic cephalosporin, and other antibiotics. Cefodizime enhanced the ingestion of anti-E IgG- coated erythrocytes by peritoneal macrophages from CD-I and BALB/cmice in a dose- dependent manner, but had no effect on uncoated or IgM-coated erythrocytes. Similar enhancement was observed only in the case of cefpimizole (AC-1370), among the other anti- biotics examined. These results suggest that the favorable in vivo activity of cefodizime and cefpimizole may result from their phagocytosis-enhancing as well as antimicrobial properties. The new semisynthetic cephalosporin, cefodizime, is characterized by a cephem ring which con- tains a ^w-methoxyimino-aminothiazolyl group at the 7-position and a thiazolylthio-methyl group at the 3-position. The latter substitution is thought to provide metabolic stability and a prolonged half-life in humanserum0. The efficacy of cefodizime in experimental murine infections is apparently superior to that predicted from in vitro activity2>3). Wepostulated that the enhanced in vivo activity of cefodizime may be due to drug-induced immunostimulation. This speculation was in part based upon the previous demonstration that a variety of agents, including lysophosphatidylcholine4) and fibronectin5), enhance receptor-dependent phagocytosis. Moreover, it was previously reported that cefpimizole (AC-1370), another semisynthetic cephalosporin, potentiated phagocyte function of macrophages and neutrophils6). -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

CP-70429, a New Penem Antibiotic
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, JUlY 1993, P. 1547-1551 Vol. 37, No. 7 0066-4804/93/071547-05$02.00/0 Copyright © 1993, American Society for Microbiology In Vitro Antibacterial Activity and ,-Lactamase Stability of CP-70,429, a New Penem Antibiotic MASASHI MINAMIMURA, YUMI TANIYAMA, EIKO INOUE,* AND SUSUMU MITSUHASHI Episome Institute, 2220 Kogure, Fujimi-mura, Seta-gun, Gunma, Japan Received 14 January 1993/Accepted 15 April 1993 In in vitro susceptibility tests, the new penem CP-70,429 showed potent antibacterial activity against gram-positive and gram-negative bacteria except Pseudomonas aeruginosa and Xanthomonas maltophilia. CP-70,429 was stable to various types of 13-lactamases except for the enzyme from X. maltophilia and was 16- to 128-fold more active than the other compounds against 13-lactamase-producing strains of Enterobacter cloacae and Citrobacterfreundii. In the past several years, many 1-lactam antibiotics with cus aureus, like the other agents against which CP-70,429 broad antibacterial activities have undergone clinical trials. was compared. CP-70,429 showed potent activity against The penem antibiotics FCE22101 and SY5555 have broad streptococci, as did the other compounds tested. Against antimicrobial spectra and high levels of stability to ,B-lacta- Enterococcus faecalis, CP-70,429 was 4- to 16-fold more mases (4, 9). The in vitro activity of CP-65,207 was previ- active than the cephems tested, on the basis of the MIC90s. ously reported by Gootz et al. (3); CP-65,207 is a mixture of CP-70,429 was the most active compound against Esche- stereoisomers of the optically pure CP-70,429. -

Of Cephalosporins. Cefuzonam (CZON, Lederle Japan, Ltd
1346 THE JOURNAL OF ANTIBIOTICS AUG. 1992 STRUCTURE-BINDING RELATIONSHIP AND BINDING SITES OF CEPHALOSPORINS IN HUMAN SERUM ALBUMIN Shuichi Tawara*, Satoru Matsumoto1, Yoshimi Matsumoto1, Toshiaki Kamimura^* and Sachiko GoTOn f New Drug Research Laboratories, Fujisawa Pharmaceutical Co., Ltd., 2-1-6 Kashima, Yodogawa-ku, Osaka 532, Japan tjDepartment of Microbiology, Toho University School of Medicine, 5-21-16 Ohmori Nishi, Ohta-ku, Tokyo 143, Japan (Received for publication January 29, 1992) The binding of some cephalosporins to human serum albumin (HSA) was studied by an ultra filtration technique. Changes in C-3 side chain resulted in marked changes in the binding to HSA,but changes in C-7 side chain did not. Cephalosporins were classified into three groups by C-3 side chain: (i) Cationic side chain with low affinity for HSA;(ii) anionic side chain with high affinity for HSA; (iii) non ionized side chain, in which binding to HSAwas dependent on lipophilicity. These findings suggest that electrostatic and hydrophobic forces play a role in the binding affinity of cephalosporins for HSA. The binding of cephalosporins with high HSAaffinity was displaced significantly by warfarin but not by phenylbutazone, L-tryptophan, or diazepam. The interaction of the cephalosporins with high affinity for HSAwith chemically modified HSAwas investigated to clarify the amino acid residues of HSAinvolved in the cephalosporin binding sites. The binding of the cephalosporins decreased remarkably with the modification of the tyrosine residues. These results suggest that the binding site of cephalosporins is located in the vicinity of warfarin binding site rather than benzodiazepine binding site and that tyrosine residues are involved in the cephalosporin binding site. -

(12) United States Patent (10) Patent No.: US 9,005,660 B2 Tygesen Et Al
USOO9005660B2 (12) United States Patent (10) Patent No.: US 9,005,660 B2 Tygesen et al. (45) Date of Patent: Apr. 14, 2015 (54) IMMEDIATE RELEASE COMPOSITION 4,873,080 A 10, 1989 Bricklet al. RESISTANT TO ABUSEBY INTAKE OF 4,892,742 A 1, 1990 Shah 4,898,733. A 2f1990 DePrince et al. ALCOHOL 5,019,396 A 5/1991 Ayer et al. 5,068,112 A 11/1991 Samejima et al. (75) Inventors: Peter Holm Tygesen, Smoerum (DK); 5,082,655 A 1/1992 Snipes et al. Jan Martin Oevergaard, Frederikssund 5,102,668 A 4, 1992 Eichel et al. 5,213,808 A 5/1993 Bar Shalom et al. (DK); Joakim Oestman, Lomma (SE) 5,266,331 A 11/1993 Oshlack et al. 5,281,420 A 1/1994 Kelmet al. (73) Assignee: Egalet Ltd., London (GB) 5,352.455 A 10, 1994 Robertson 5,411,745 A 5/1995 Oshlack et al. (*) Notice: Subject to any disclaimer, the term of this 5,419,917 A 5/1995 Chen et al. patent is extended or adjusted under 35 5,422,123 A 6/1995 Conte et al. U.S.C. 154(b) by 473 days. 5,460,826 A 10, 1995 Merrill et al. 5,478,577 A 12/1995 Sackler et al. 5,508,042 A 4/1996 OShlack et al. (21) Appl. No.: 12/701.248 5,520,931 A 5/1996 Persson et al. 5,529,787 A 6/1996 Merrill et al. (22) Filed: Feb. 5, 2010 5,549,912 A 8, 1996 OShlack et al. -

Polyanhydride-Based Nanomedicine Platform for Combating Neurodegeneration Benjamin W
Polyanhydride-based Nanomedicine Platform for Combating Neurodegeneration Benjamin W. Schlichtmann1, Shivani Ghaisas2, Vellareddy Anantharam2, Anumantha Kanthasamy2, Surya Mallapragada1, and Balaji Narasimhan1. 1Chemical & Biological Engineering and 2Biomedical Sciences, Iowa State University, Ames, IA 50011 Statement of Purpose: Treatment for exposure to (blue arrows). The average NP size of NF-NPs and CPTP- chemical toxins such as pesticides has resulted in an NPs was 397 ± 143 nm and 287 ± 99 nm, respectively, estimated annual cost of $200 million in recent years [1]. which is in agreement with previous work [4]. Surface Exposure to these toxins leads to neurodegeneration, zeta potential measurements demonstrated a negligible including development of chronic conditions such as effect of functionalization on surface charge, as expected. Parkinson’s and Alzheimer’s diseases. Drugs that combat Non-functionalized neurodegeneration, such as antioxidants, must effectively pass through the blood-brain barrier (BBB), and then target diseased neurons and their mitochondria. The efficacy of drugs administered alone to do so is significantly hindered by drug metabolism and limited CPTP bulk-functionalized localization. Polyanhydride nanoparticles (NPs) have several favorable characteristics for drug delivery to overcome these issues and improve treatment of neurodegeneration [2]. Localization can be further Pre-CPTP (control) improved using targeting ligands by directly functionalizing the polyanhydrides to ensure that the ligand persists throughout NP degradation. The lipophilic cationic ligand triphenylphosphonium (TPP) has recently shown enhanced targeting of the BBB and mitochondria Figure 1. FTIR of functionalized and non-functionalized [3]. In this work, a derivative of TPP, (3-carboxypropyl) polymer showing functionalization by the relative triphenylphosphonium (CPTP) was used to functionalize decrease (red) of non-functionalized polymer end-group polyanhydrides. -

Consideration of Antibacterial Medicines As Part Of
Consideration of antibacterial medicines as part of the revisions to 2019 WHO Model List of Essential Medicines for adults (EML) and Model List of Essential Medicines for children (EMLc) Section 6.2 Antibacterials including Access, Watch and Reserve Lists of antibiotics This summary has been prepared by the Health Technologies and Pharmaceuticals (HTP) programme at the WHO Regional Office for Europe. It is intended to communicate changes to the 2019 WHO Model List of Essential Medicines for adults (EML) and Model List of Essential Medicines for children (EMLc) to national counterparts involved in the evidence-based selection of medicines for inclusion in national essential medicines lists (NEMLs), lists of medicines for inclusion in reimbursement programs, and medicine formularies for use in primary, secondary and tertiary care. This document does not replace the full report of the WHO Expert Committee on Selection and Use of Essential Medicines (see The selection and use of essential medicines: report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2019 (including the 21st WHO Model List of Essential Medicines and the 7th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization; 2019 (WHO Technical Report Series, No. 1021). Licence: CC BY-NC-SA 3.0 IGO: https://apps.who.int/iris/bitstream/handle/10665/330668/9789241210300-eng.pdf?ua=1) and Corrigenda (March 2020) – TRS1021 (https://www.who.int/medicines/publications/essentialmedicines/TRS1021_corrigenda_March2020. pdf?ua=1). Executive summary of the report: https://apps.who.int/iris/bitstream/handle/10665/325773/WHO- MVP-EMP-IAU-2019.05-eng.pdf?ua=1. -

Pharmaceutical Appendix to the Harmonized Tariff Schedule
Harmonized Tariff Schedule of the United States (2019) Revision 13 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2019) Revision 13 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known.