Gastropoda: Pulmonata: Vertiginidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Pulmonata: Vertiginidae) and Strobilops
Records of the Hawaii Biological Survey for 2012. Edited by Neal L. Evenhuis & Lucius G. Eldredge. Bishop Museum Occasional Papers 114: 39 –42 (2013) Hawaiian land snail records : Lyropupa cookei Clench , 1952 (Pulmonata : Vertiginidae ) and Strobilops aeneus Pilsbry , 1926 (Pulmonata : Strobilopsidae ) CARl C. C HRiSTeNSeN Bishop Museum, 1525 Bernice Street, Honolulu, Hawai‘i 96817-2704, USA; email: [email protected] This note clarifies the status of two taxa of land snails that have been reported to occur in the Hawaiian islands. Lyropupa cookei Clench, 1952, is shown to be a synonym of Lyropupa anceyana Cooke & Pilsbry in Pilsbry & Cooke, 1920. The sole Hawaiian record for the North American Strobilops aeneus Pilsbry, 1926, is almost certainly based on a mislabeled specimen, and accordingly this species should be removed from the Hawaiian faunal list. Lyropupa cookei Clench , 1952 Lyropupa Pilsbry, 1900, is a genus of pupilloid land snails endemic to the Hawaiian islands. in their monograph of the genus, Pilsbry & Cooke (1920 in 1918–1920: 253–254, pl. 26, figs. 3, 6) published a description of “ Lyropupa anceyana C. & P., n. sp.,” based on specimens from ola‘a on the island of Hawai‘i held in the collections of Bishop Museum and the Academy of Natural Sciences of Philadelphia. They stated that their new species had previously been misidentified by Ancey (1904:124) as Lyropupa lyrata (Gould, 1843) . Several pages earlier, in their systematic treatment of that species, Pilsbry & Cooke (1918–1920: 235) had also set forth their conclusion that Ancey had misidenti - fied Gould’s species and stated that in fact Ancey’s “description of lyrata was based on specimens of an unnamed species for which the name L. -

Surveys for Desmoulin's Whorl Snail Vertigo Moulinsiana on Cors Geirch
Surveys for Desmoulin’s Whorl Snail Vertigo moulinsiana on Cors Geirch NNR/SSSI and the Afon Penrhos floodplain & for Geyer’s Whorl Snail Vertigo geyeri on Cors Geirch NNR in 2017 Martin Willing NRW Evidence Report No. 258 D8 Figure1. Newly discovered Vertigo moulinsiana habitat at Afon Penrhos. NRW Evidence Report No. 258 About Natural Resources Wales Natural Resources Wales is the organisation responsible for the work carried out by the three former organisations, the Countryside Council for Wales, Environment Agency Wales and Forestry Commission Wales. It is also responsible for some functions previously undertaken by Welsh Government. Our purpose is to ensure that the natural resources of Wales are sustainably maintained, used and enhanced, now and in the future. We work for the communities of Wales to protect people and their homes as much as possible from environmental incidents like flooding and pollution. We provide opportunities for people to learn, use and benefit from Wales' natural resources. We work to support Wales' economy by enabling the sustainable use of natural resources to support jobs and enterprise. We help businesses and developers to understand and consider environmental limits when they make important decisions. We work to maintain and improve the quality of the environment for everyone and we work towards making the environment and our natural resources more resilient to climate change and other pressures. Evidence at Natural Resources Wales Natural Resources Wales is an evidence based organisation. We seek to ensure that our strategy, decisions, operations and advice to Welsh Government and others are underpinned by sound and quality-assured evidence. -

S1016 Vertigo Moulinsiana Desmoulin's Whorl Snail
European Community Directive on the Conservation of Natural Habitats and of Wild Fauna and Flora (92/43/EEC) Second Report by the United Kingdom under Article 17 on the implementation of the Directive from January 2001 to December 2006 Conservation status assessment for : S1016: Vertigo moulinsiana - Desmoulin's whorl snail Please note that this is a section of the report. For the complete report visit http://www.jncc.gov.uk/article17 Please cite as: Joint Nature Conservation Committee. 2007. Second Report by the UK under Article 17 on the implementation of the Habitats Directive from January 2001 to December 2006. Peterborough: JNCC. Available from: www.jncc.gov.uk/article17 Second Report by the United Kingdom under Article 17 on the implementation of the Directive from January 2001 to December 2006 S1016 Vertigo moulinsiana Desmoulin’s Whorl Snail Audit trail compiled and edited by JNCC and the Invertebrate Inter-Agency Working Group This document is an audit of the data and judgements on conservation status in the UK’s report on the implementation of the Habitats Directive (January 2001 to December 2006) for this species. Superscript numbers accompanying the headings below, cross-reference to headings in the corresponding Annex B reporting form. This supporting information should be read in conjunction with the UK approach for species (see ‘Assessing Conservation Status: UK Approach’). 1. Range Information2.3 1.1 Surface area of range2.3.1 17,466 km2 The above estimate was calculated using records collected from 1990 onwards within the Alpha Hull software. Extent of occurrence was used as a proxy measure for range (see Map 1.1), and a 10km resolution was assumed. -

Pupillid Land Snails of Eastern North America*
Amer. Malac. Bull. 28: 1-29 (2010) Pupillid land snails of eastern North America* Jeffrey C. Nekola1 and Brian F. Coles2 1 Department of Biology, University of New Mexico, Albuquerque, New Mexico 87131, U.S.A. 2 Mollusca Section, Department of Biodiversity, National Museum of Wales, Cathays Park, Cardiff CF10 3NP, U.K. Corresponding author: [email protected] Abstract: The Pupillidae form an important component of eastern North American land snail biodiversity, representing approx. 12% of the entire fauna, 25-75% of all species and individuals at regional scales, at least 30% of the species diversity, and 33% of individuals within any given site. In some regions pupillids represent 80-100% of total molluscan diversity within sites, notably in taiga, tundra, and the base-poor pine savannas and pocosins of the southeastern coastal plain. Adequate documentation of North American land snail biodiversity thus requires investigators to effi ciently collect and accurately identify individuals of this group. This paper presents a set of annotated keys to the 65 species in this family known to occur in North America east of the Rocky Mountains. The distinguishing taxonomic features, updated county-scale range maps, and ecological conditions favored by each are presented in hopes of stimulating future research in this important group. Key words: microsnail, biodiversity, ecology, biogeography, taxonomy For the last dozen years, our interests in terrestrial Adequate documentation of this diversity thus requires gastropod biodiversity have lead us individually and investigators to effi ciently collect and accurately identify collectively to observe molluscan communities over most of individuals from this family. Unfortunately, neither has been North America, ranging from central Quebec, Hudson’s Bay common. -

Guidance Document on the Strict Protection of Animal Species of Community Interest Under the Habitats Directive 92/43/EEC
Guidance document on the strict protection of animal species of Community interest under the Habitats Directive 92/43/EEC Final version, February 2007 1 TABLE OF CONTENTS FOREWORD 4 I. CONTEXT 6 I.1 Species conservation within a wider legal and political context 6 I.1.1 Political context 6 I.1.2 Legal context 7 I.2 Species conservation within the overall scheme of Directive 92/43/EEC 8 I.2.1 Primary aim of the Directive: the role of Article 2 8 I.2.2 Favourable conservation status 9 I.2.3 Species conservation instruments 11 I.2.3.a) The Annexes 13 I.2.3.b) The protection of animal species listed under both Annexes II and IV in Natura 2000 sites 15 I.2.4 Basic principles of species conservation 17 I.2.4.a) Good knowledge and surveillance of conservation status 17 I.2.4.b) Appropriate and effective character of measures taken 19 II. ARTICLE 12 23 II.1 General legal considerations 23 II.2 Requisite measures for a system of strict protection 26 II.2.1 Measures to establish and effectively implement a system of strict protection 26 II.2.2 Measures to ensure favourable conservation status 27 II.2.3 Measures regarding the situations described in Article 12 28 II.2.4 Provisions of Article 12(1)(a)-(d) in relation to ongoing activities 30 II.3 The specific protection provisions under Article 12 35 II.3.1 Deliberate capture or killing of specimens of Annex IV(a) species 35 II.3.2 Deliberate disturbance of Annex IV(a) species, particularly during periods of breeding, rearing, hibernation and migration 37 II.3.2.a) Disturbance 37 II.3.2.b) Periods -

Le Tuf Calcaire De La Celle-Sur-Seine (Seine Et Marne) : Nouvelles Données Sur Un Site Clé Du Stade 11 Dans Le Nord De La France ⅲ
Quaternaire, 17, (2), 2006, p. 5-29 LE TUF CALCAIRE DE LA CELLE-SUR-SEINE (SEINE ET MARNE) : NOUVELLES DONNÉES SUR UN SITE CLÉ DU STADE 11 DANS LE NORD DE LA FRANCE Ⅲ N. LIMONDIN-LOZOUET1, P. ANTOINE1, P. AUGUSTE2, J.J. BAHAIN3, P. CARBONEL4, C. CHAUSSÉ1, N. CONNET5, J. DUPERON6, M. DUPERON6, C. FALGUERES3, P. FREYTET7, B. GHALEB8, M.C. JOLLY-SAAD9, V. LHOMME5, P. LOZOUET10, N. MERCIER11, J.F. PASTRE1 & P. VOINCHET3 RÉSUMÉ De nouvelles études morphostratigraphiques et biostratigraphiques ainsi que des datations ont été entreprises sur le célèbre dépôt de tuf cal- caire de La Celle-sur-Seine afin d’obtenir une reconstruction détaillée des successions paléoenvironnementales et climatiques enregistrées dans cette formation interglaciaire du Pléistocène moyen. L’approche développée a privilégié l’aspect pluridisciplinaire afin d’exploiter la richesse pa- léontologique du gisement, de mieux appréhender ses caractères morphostratigraphiques et de préciser sa position chronologique. Les premiers résultats obtenus à partir des études malacologiques montrent que le dépôt, composé de tufs et de niveaux limono-argileux, s’est construit en progradant le long du versant. Ainsi l’épaisseur totale de la formation atteint près de 9 mètres. Les malacofaunes permettent de re- constituer une évolution paléoenvironnementale correspondant au début puis à l’optimum d’une phase climatique interglaciaire. Les niveaux som- mitaux sont, eux, caractérisés par un net recul de la couverture forestière. La création de nouveaux profils stratigraphiques a permis la découverte dans un niveau de limon gris tufacé d’une abondante faune de mam- mifères accompagnée par quelques silex taillés. Cet horizon appartient à la malacozone la plus riche en taxons thermophiles qui est interprétée comme la phase optimum de l’Interglaciaire. -
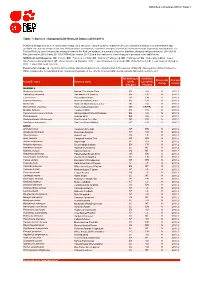
Table 7: Species Changing IUCN Red List Status (2010-2011)
IUCN Red List version 2011.2: Table 7 Table 7: Species changing IUCN Red List Status (2010-2011) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2010 (IUCN Red List version 2010.4) and 2011 (IUCN Red List version 2011.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2010) List (2011) change version Category Category MAMMALS Bradypus torquatus Maned Three-toed Sloth EN VU N 2011.1 Callicebus oenanthe San Martin Titi Monkey EN CR N 2011.1 Equus ferus Przewalski's Horse CR EN G 2011.2 -

European Glacial Relict Snails and Plants: Environmental Context of Their Modern Refugial Occurrence in Southern Siberia
bs_bs_banner European glacial relict snails and plants: environmental context of their modern refugial occurrence in southern Siberia MICHAL HORSAK, MILAN CHYTRY, PETRA HAJKOV A, MICHAL HAJEK, JIRI DANIHELKA, VERONIKA HORSAKOV A, NIKOLAI ERMAKOV, DMITRY A. GERMAN, MARTIN KOCI, PAVEL LUSTYK, JEFFREY C. NEKOLA, ZDENKA PREISLEROVA AND MILAN VALACHOVIC Horsak, M., Chytry, M., Hajkov a, P., Hajek, M., Danihelka, J., Horsakov a,V.,Ermakov,N.,German,D.A.,Ko cı, M., Lustyk, P., Nekola, J. C., Preislerova, Z. & Valachovic, M. 2015 (October): European glacial relict snails and plants: environmental context of their modern refugial occurrence in southern Siberia. Boreas, Vol. 44, pp. 638–657. 10.1111/bor.12133. ISSN 0300-9483. Knowledge of present-day communities and ecosystems resembling those reconstructed from the fossil record can help improve our understanding of historical distribution patterns and species composition of past communities. Here, we use a unique data set of 570 plots explored for vascular plant and 315 for land-snail assemblages located along a 650-km-long transect running across a steep climatic gradient in the Russian Altai Mountains and their foothills in southern Siberia. We analysed climatic and habitat requirements of modern populations for eight land-snail and 16 vascular plant species that are considered characteristic of the full-glacial environment of central Europe based on (i) fossil evidence from loess deposits (snails) or (ii) refugial patterns of their modern distribu- tions (plants). The analysis yielded consistent predictions of the full-glacial central European climate derived from both snail and plant populations. We found that the distribution of these 24 species was limited to the areas with mean annual temperature varying from À6.7 to 3.4 °C (median À2.5 °C) and with total annual precipitation vary- ing from 137 to 593 mm (median 283 mm). -

Palaeoenvironmental Dynamics of the MIS 11 Interglacial in North-Western
Palaeoenvironmental dynamics of the MIS 11 interglacial in north-western Europe based on the malacological succession from La Celle (Seine Valley, France): Relationship with glacial refugia and palaeobiodiversity Nicole Limondin-Lozouet, Julie Dabkowski, Pierre Antoine To cite this version: Nicole Limondin-Lozouet, Julie Dabkowski, Pierre Antoine. Palaeoenvironmental dynamics of the MIS 11 interglacial in north-western Europe based on the malacological succession from La Celle (Seine Valley, France): Relationship with glacial refugia and palaeobiodiversity. Palaeogeography, Palaeoclimatology, Palaeoecology, Elsevier, 2020, 560, pp.110044. 10.1016/j.palaeo.2020.110044. hal-02990686 HAL Id: hal-02990686 https://hal.archives-ouvertes.fr/hal-02990686 Submitted on 31 Aug 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Palaeoenvironmental dynamics of the MIS 11 interglacial in north-western Europe based on the malacological succession from La Celle (Seine Valley, France): Relationship with glacial refugia and palaeobiodiversity Nicole Limondin-Lozouet, Julie Dabkowski, Pierre Antoine To cite this version: Nicole Limondin-Lozouet, Julie Dabkowski, Pierre Antoine. Palaeoenvironmental dynamics of the MIS 11 interglacial in north-western Europe based on the malacological succession from La Celle (Seine Valley, France): Relationship with glacial refugia and palaeobiodiversity. -

Fauna of New Zealand Website Copy 2010, Fnz.Landcareresearch.Co.Nz
aua o ew eaa Ko te Aiaga eeke o Aoeaoa IEEAE SYSEMAICS AISOY GOU EESEAIES O ACAE ESEAC ema acae eseac ico Agicuue & Sciece Cee P O o 9 ico ew eaa K Cosy a M-C aiièe acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa EESEAIE O UIESIIES M Emeso eame o Eomoogy & Aima Ecoogy PO o ico Uiesiy ew eaa EESEAIE O MUSEUMS M ama aua Eiome eame Museum o ew eaa e aa ogaewa O o 7 Weigo ew eaa EESEAIE O OESEAS ISIUIOS awece CSIO iisio o Eomoogy GO o 17 Caea Ciy AC 1 Ausaia SEIES EIO AUA O EW EAA M C ua (ecease ue 199 acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa Fauna of New Zealand Ko te Aitanga Pepeke o Aotearoa Number / Nama 38 Naturalised terrestrial Stylommatophora (Mousca Gasooa Gay M ake acae eseac iae ag 317 amio ew eaa 4 Maaaki Whenua Ρ Ε S S ico Caeuy ew eaa 1999 Coyig © acae eseac ew eaa 1999 o a o is wok coee y coyig may e eouce o coie i ay om o y ay meas (gaic eecoic o mecaica icuig oocoyig ecoig aig iomaio eiea sysems o oewise wiou e wie emissio o e uise Caaoguig i uicaio AKE G Μ (Gay Micae 195— auase eesia Syommaooa (Mousca Gasooa / G Μ ake — ico Caeuy Maaaki Weua ess 1999 (aua o ew eaa ISS 111-533 ; o 3 IS -7-93-5 I ie 11 Seies UC 593(931 eae o uIicaio y e seies eio (a comee y eo Cosy usig comue-ase e ocessig ayou scaig a iig a acae eseac M Ae eseac Cee iae ag 917 Aucka ew eaa Māoi summay e y aco uaau Cosuas Weigo uise y Maaaki Weua ess acae eseac O o ico Caeuy Wesie //wwwmwessco/ ie y G i Weigo o coe eoceas eicuaum (ue a eigo oaa (owe (IIusao G M ake oucio o e coou Iaes was ue y e ew eaIa oey oa ue oeies eseac -

Guidelines for the Capture and Management of Digital Zoological Names Information Francisco W
Guidelines for the Capture and Management of Digital Zoological Names Information Francisco W. Welter-Schultes Version 1.1 March 2013 Suggested citation: Welter-Schultes, F.W. (2012). Guidelines for the capture and management of digital zoological names information. Version 1.1 released on March 2013. Copenhagen: Global Biodiversity Information Facility, 126 pp, ISBN: 87-92020-44-5, accessible online at http://www.gbif.org/orc/?doc_id=2784. ISBN: 87-92020-44-5 (10 digits), 978-87-92020-44-4 (13 digits). Persistent URI: http://www.gbif.org/orc/?doc_id=2784. Language: English. Copyright © F. W. Welter-Schultes & Global Biodiversity Information Facility, 2012. Disclaimer: The information, ideas, and opinions presented in this publication are those of the author and do not represent those of GBIF. License: This document is licensed under Creative Commons Attribution 3.0. Document Control: Version Description Date of release Author(s) 0.1 First complete draft. January 2012 F. W. Welter- Schultes 0.2 Document re-structured to improve February 2012 F. W. Welter- usability. Available for public Schultes & A. review. González-Talaván 1.0 First public version of the June 2012 F. W. Welter- document. Schultes 1.1 Minor editions March 2013 F. W. Welter- Schultes Cover Credit: GBIF Secretariat, 2012. Image by Levi Szekeres (Romania), obtained by stock.xchng (http://www.sxc.hu/photo/1389360). March 2013 ii Guidelines for the management of digital zoological names information Version 1.1 Table of Contents How to use this book ......................................................................... 1 SECTION I 1. Introduction ................................................................................ 2 1.1. Identifiers and the role of Linnean names ......................................... 2 1.1.1 Identifiers .................................................................................. -

The Canadian Field-Naturalist
The Canadian Field-Naturalist Tall grass prairie ecosystem management—a gastropod perspective Annegret Nicolai1, 2, *, Robert G. Forsyth3, Melissa Grantham4, and Cary D. Hamel4 1Université Rennes, UMR CNRS 6553 EcoBio, Station Biologique Paimpont, Paimpont 35380 France 2Western University, Department of Biology, 1151 Richmond Street North, London, Ontario N6A 5B7 Canada 3New Brunswick Museum, 277 Douglas Avenue, Saint John, New Brunswick E2K 1E5 Canada 4Nature Conservancy of Canada, Manitoba Region, Suite 200 - 611 Corydon Avenue, Winnipeg, Manitoba R3L 0P3 Canada *Corresponding author: [email protected] Nicolai, A., R.G. Forsyth, M. Grantham, and C.D. Hamel. 2019. Tall grass prairie ecosystem management—a gastropod perspective. Canadian Field-Naturalist 133(4): 313–324. https://doi.org/10.22621/cfn.v133i4.2217 Abstract Less than 5% of the original tall grass prairie in North America remains. A portion of this remnant, composed of wetland, grassland and forest, is protected by the Nature Conservancy of Canada (NCC) in southern Manitoba. This heterogene- ous ecosystem has rich biodiversity; however, gastropods have not been surveyed in Canada’s tall grass prairie. We studied gastropods in Prairie, Wet Meadow, Forest, and Wet Forest habitats of the Manitoba Tall Grass Prairie Preserve that vary with respect to land management practices (prescribed burning, grazing by cattle). Gastropod community composition was unique in the Prairie where mounds of grass litter form permanently moist cavities harbouring aquatic species, while dry-habitat species colonized the upper parts of these mounds. Gastropod communities in Prairie habitats were negatively affected by grazing and burning that occurred in the five years prior to our survey.