1790LS Pre. Pages.P65
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Successful Candidates
11 - LIST OF SUCCESSFUL CANDIDATES CONSTITUENCY WINNER PARTY Andhra Pradesh 1 Nagarkurnool Dr. Manda Jagannath INC 2 Nalgonda Gutha Sukender Reddy INC 3 Bhongir Komatireddy Raj Gopal Reddy INC 4 Warangal Rajaiah Siricilla INC 5 Mahabubabad P. Balram INC 6 Khammam Nama Nageswara Rao TDP 7 Aruku Kishore Chandra Suryanarayana INC Deo Vyricherla 8 Srikakulam Killi Krupa Rani INC 9 Vizianagaram Jhansi Lakshmi Botcha INC 10 Visakhapatnam Daggubati Purandeswari INC 11 Anakapalli Sabbam Hari INC 12 Kakinada M.M.Pallamraju INC 13 Amalapuram G.V.Harsha Kumar INC 14 Rajahmundry Aruna Kumar Vundavalli INC 15 Narsapuram Bapiraju Kanumuru INC 16 Eluru Kavuri Sambasiva Rao INC 17 Machilipatnam Konakalla Narayana Rao TDP 18 Vijayawada Lagadapati Raja Gopal INC 19 Guntur Rayapati Sambasiva Rao INC 20 Narasaraopet Modugula Venugopala Reddy TDP 21 Bapatla Panabaka Lakshmi INC 22 Ongole Magunta Srinivasulu Reddy INC 23 Nandyal S.P.Y.Reddy INC 24 Kurnool Kotla Jaya Surya Prakash Reddy INC 25 Anantapur Anantha Venkata Rami Reddy INC 26 Hindupur Kristappa Nimmala TDP 27 Kadapa Y.S. Jagan Mohan Reddy INC 28 Nellore Mekapati Rajamohan Reddy INC 29 Tirupati Chinta Mohan INC 30 Rajampet Annayyagari Sai Prathap INC 31 Chittoor Naramalli Sivaprasad TDP 32 Adilabad Rathod Ramesh TDP 33 Peddapalle Dr.G.Vivekanand INC 34 Karimnagar Ponnam Prabhakar INC 35 Nizamabad Madhu Yaskhi Goud INC 36 Zahirabad Suresh Kumar Shetkar INC 37 Medak Vijaya Shanthi .M TRS 38 Malkajgiri Sarvey Sathyanarayana INC 39 Secundrabad Anjan Kumar Yadav M INC 40 Hyderabad Asaduddin Owaisi AIMIM 41 Chelvella Jaipal Reddy Sudini INC 1 GENERAL ELECTIONS,INDIA 2009 LIST OF SUCCESSFUL CANDIDATE CONSTITUENCY WINNER PARTY Andhra Pradesh 42 Mahbubnagar K. -
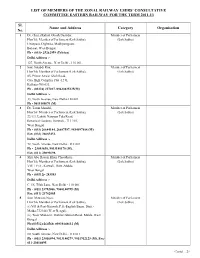
List of Members of the Zonal Railway Users’ Consultative Committee Eastern Railway for the Term 2011-13
LIST OF MEMBERS OF THE ZONAL RAILWAY USERS’ CONSULTATIVE COMMITTEE EASTERN RAILWAY FOR THE TERM 2011-13 Sl. Name and Address Category Organisation No. 1 Dr. (Smt.) Kakoli Ghosh Dastidar, Member of Parliament Hon’ble Member of Parliament (Lok Sabha), (Lok Sabha) Uttarpara, Digberia, Madhyamgram, Barasat, West Bengal. Ph - (033)- 25262959 (Telefax) Delhi Address :- 127, North Avenue, New Delhi - 110 001. 2 Smt. Satabdi Roy, Member of Parliament Hon’ble Member of Parliament (Lok Sabha), (Lok Sabha) 85, Prince Anwar Shah Road, City High Complex Flat -12 G, Kolkata-700 033. Ph - (03324) 227017, 09433025125(M) Delhi Address :- 33, North Avenue, New Delhi-110 001. Ph - 9013180070 (M) 3 Dr. Tarun Mandal, Member of Parliament Hon’ble Member of Parliament (Lok Sabha), (Lok Sabha) 22/1/3, Lakshi Narayan Tala Road, Botanical Gardens, Howrah - 711 103, West Bengal. Ph - (033) 26684184, 26687597, 9434097888 (M) Fax. (033) 26685451. Delhi Address :- 72, North Avenue, New Delhi - 110 001. Ph - 23093658, 9013180170 (M), Fax. (011) 23093698. 4 Shri Abu Hasem Khan Choudhury, Member of Parliament Hon’ble Member of Parliament (Lok Sabha), (Lok Sabha) Vill + P.O - Kotwali, Distt.-Malda, West Bengal. Ph - (03512)- 283593 Delhi Address :- C 1/6, Tilak Lane, New Delhi - 110 001. Ph - (011) 23782066, 9868180995 (M) Fax. (011) 23782065 5 Smt. Mausam Noor, Member of Parliament Hon’ble Member of Parliament (Lok Sabha), (Lok Sabha) (i) Vill & Post-Kotwali, P.S.-English Bazar, Distt.- Malda-732144 (West Bengal). (ii) 'Noor Mansion', Rathlari Station Road, Malda, West Bengal. Ph-(03512)-264560, 09830448612 (M) Delhi Address :- 80, South Avenue, New Delhi - 110 011. -

LOK SABHA ___ BULLETIN-PART II (General Information Relating To
LOK SABHA ___ BULLETIN-PART II (General Information relating to Parliamentary and other matters) ________________________________________________________________________ Nos. 7315-7338] [Tuesday, Septemeber 18, 2018/ Bhadrapada 27, 1940(Saka) _________________________________________________________________________ No. 7315 Committee Branch-I Members of the Committee on Commerce The following are the members of the Committee on Commerce w.e.f. 01 September, 2018:- Lok Sabha 1. Shri Dibyendu Adhikari 2. Shri Subhash Chandra Baheria 3. Shri Abhishek Banerjee 4. Smt. Bijoya Chakravarty 5. Shri Jitendra Chaudhury 6. Shri Dushyant Chautala 7. Smt. Kavitha Kalvakuntla 8. Dr. Hari Babu Kambhampati 9. Shri Nityanand Rai 10. Shri Dhananjay Bhimrao Mahadik 11. Shri Kamal Nath 12. Shri Kamlesh Paswan 13. Shri K.R.P. Prabakaran 14. Shri T. Radhakrishnan 15. Shri Dipsinh Shankarsinh Rathod 16. Shri Khan Saumitra 17. Advocate (Shri) Narendra Keshav Sawaikar 18. Shri Kirti Vardhan Singh 19. Shri Vinod Kumar Sonkar 20. Shri Narsimham Thota 21. Vacant Rajya Sabha 22. Smt. Roopa Ganguly 23. Shri Naresh Gujral 24. Shri Sushil Kumar Gupta 25. Shri Ram Kumar Kashyap 26. Shri M.P. Veerendra Kumar 27. Smt. Thota Seetharama Lakshmi 28. Shri Vayalar Ravi 29. Shri Kapil Sibal 30. Dr. Kanwar Deep Singh 31. Shri Rakesh Sinha Shri Naresh Gujral has been appointed Chairperson of the Committee. ---------- No.7316 Committee Branch-I Members of the Committee on Home Affairs The following are the members of the Committee on Home Affairs w.e.f. 01 September, 2018:- Lok Sabha 1. Dr. Sanjeev Kumar Balyan 2. Shri Prem Singh Chandumajra 3. Shri Adhir Ranjan Chowdhury 4. Dr. (Smt.) Kakoli Ghosh Dastidar 5. Shri Ramen Deka 6. -

List of Winning Candidated Final for 16Th
Leading/Winning State PC No PC Name Candidate Leading/Winning Party Andhra Pradesh 1 Adilabad Rathod Ramesh Telugu Desam Andhra Pradesh 2 Peddapalle Dr.G.Vivekanand Indian National Congress Andhra Pradesh 3 Karimnagar Ponnam Prabhakar Indian National Congress Andhra Pradesh 4 Nizamabad Madhu Yaskhi Goud Indian National Congress Andhra Pradesh 5 Zahirabad Suresh Kumar Shetkar Indian National Congress Andhra Pradesh 6 Medak Vijaya Shanthi .M Telangana Rashtra Samithi Andhra Pradesh 7 Malkajgiri Sarvey Sathyanarayana Indian National Congress Andhra Pradesh 8 Secundrabad Anjan Kumar Yadav M Indian National Congress Andhra Pradesh 9 Hyderabad Asaduddin Owaisi All India Majlis-E-Ittehadul Muslimeen Andhra Pradesh 10 Chelvella Jaipal Reddy Sudini Indian National Congress Andhra Pradesh 11 Mahbubnagar K. Chandrasekhar Rao Telangana Rashtra Samithi Andhra Pradesh 12 Nagarkurnool Dr. Manda Jagannath Indian National Congress Andhra Pradesh 13 Nalgonda Gutha Sukender Reddy Indian National Congress Andhra Pradesh 14 Bhongir Komatireddy Raj Gopal Reddy Indian National Congress Andhra Pradesh 15 Warangal Rajaiah Siricilla Indian National Congress Andhra Pradesh 16 Mahabubabad P. Balram Indian National Congress Andhra Pradesh 17 Khammam Nama Nageswara Rao Telugu Desam Kishore Chandra Suryanarayana Andhra Pradesh 18 Aruku Deo Vyricherla Indian National Congress Andhra Pradesh 19 Srikakulam Killi Krupa Rani Indian National Congress Andhra Pradesh 20 Vizianagaram Jhansi Lakshmi Botcha Indian National Congress Andhra Pradesh 21 Visakhapatnam Daggubati Purandeswari -

4 Patrimonial and Programmatic Talking About Democracy in a South Indian Village
4 Patrimonial and Programmatic Talking about Democracy in a South Indian Village PAMELA PRICE AND DUSI SRINIVAS How do people in India participate politically, as citizens, clients and/or subjects?1 This query appears in various forms in ongoing debates concerning the extent and nature of civil society, the pitfalls of patronage democracy, and the role of illegal- ity in political practice, to name a few of the several concerns about political spheres in India. A focus for discussion has been the relationship of civil society institutions (with associated principles of equality and fairness) to political spheres driven mainly by political parties and to what Partha Chatterjee desig- nated as ‘political society’.2 Since 2005, with the publication of the monograph, Seeing the State: Governance and Governmentality in India (Corbridge et al.), there is growing support for the argument that political cultures and practices in India, from place to place and time to time, to greater and lesser degrees, include 1. Thanks to those who commented on earlier drafts of this piece when it was presented at the Department of Political Science at the University of Hyderabad, the South Asia Symposium in Oslo, and at the workshop ‘Practices and Experiences of Democracy in Post-colonial Locali- ties’, part of the conference, ‘Democracy as Idea and Practice’ organized by the University of Oslo. We are grateful to K.C. Suri for suggesting the term ‘programmatic’ in our discussions of the findings here. Thanks to the editors of this volume, David Gilmartin and Sten Widmalm for reading and commenting on this piece. -
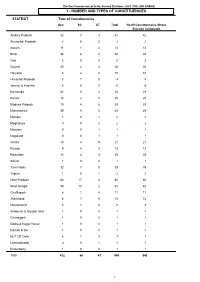
Number and Types of Constituencies
Election Commission of India, General Elections, 2009 (15th LOK SABHA) 3 - NUMBER AND TYPES OF CONSTITUENCIES STATE/UT Type of Constituencies Gen SC ST Total No Of Constituencies Where Election Completed Andhra Pradesh 32 7 3 42 42 Arunachal Pradesh 2 0 0 2 2 Assam 11 1 2 14 14 Bihar 34 6 0 40 40 Goa 2 0 0 2 2 Gujarat 20 2 4 26 26 Haryana 8 2 0 10 10 Himachal Pradesh 3 1 0 4 4 Jammu & Kashmir 6 0 0 6 6 Karnataka 21 5 2 28 28 Kerala 18 2 0 20 20 Madhya Pradesh 19 4 6 29 29 Maharashtra 39 5 4 48 48 Manipur 1 0 1 2 2 Meghalaya 0 0 2 2 2 Mizoram 0 0 1 1 1 Nagaland 0 0 1 1 1 Orissa 13 3 5 21 21 Punjab 9 4 0 13 13 Rajasthan 18 4 3 25 25 Sikkim 1 0 0 1 1 Tamil Nadu 32 7 0 39 39 Tripura 1 0 1 2 2 Uttar Pradesh 63 17 0 80 80 West Bengal 30 10 2 42 42 Chattisgarh 6 1 4 11 11 Jharkhand 8 1 5 14 14 Uttarakhand 4 1 0 5 5 Andaman & Nicobar Islands 1 0 0 1 1 Chandigarh 1 0 0 1 1 Dadra & Nagar Haveli 1 0 0 1 1 Daman & Diu 1 0 0 1 1 NCT OF Delhi 6 1 0 7 7 Lakshadweep 0 0 1 1 1 Puducherry 1 0 0 1 1 Total 412 84 47 543 543 1 Election Commission of India, General Elections, 2009 (15th LOK SABHA) 4 - POLLING STATION INFORMATION Polling Stations General Electors Service Electors Grand Total State/UT PC No. -

Parliamentary Bulletin
RAJYA SABHA Parliamentary Bulletin PART-II Nos.: 51236-51237] WEDNESDAY, SEPTEMBER 4, 2013 No. 51236 Committee Co-ordination Section Meeting of the Parliamentary Forum on Youth As intimated by the Lok Sabha Secretariat, a meeting of the Parliamentary Forum on Youth on the subject ‘Youth and Social Media: Challenges and Opportunities’ will be held on Thursday, 05 September, 2013 at 1530 hrs. in Committee Room No.074, Ground Floor, Parliament Library Building, New Delhi. Shri Naman Pugalia, Public Affairs Analyst, Google India will make a presentation. 2. Members are requested to kindly make it convenient to attend the meeting. ——— No. 51237 Committee Co-ordination Section Re-constitution of the Department-related Parliamentary Standing Committees (2013-2014) The Department–related Parliamentary Standing Committees have been reconstituted w.e.f. 31st August, 2013 as follows: - Committee on Commerce RAJYA SABHA 1. Shri Birendra Prasad Baishya 2. Shri K.N. Balagopal 3. Shri P. Bhattacharya 4. Shri Shadi Lal Batra 2 5. Shri Vijay Jawaharlal Darda 6. Shri Prem Chand Gupta 7. Shri Ishwarlal Shankarlal Jain 8. Shri Shanta Kumar 9. Dr. Vijay Mallya 10. Shri Rangasayee Ramakrishna LOK SABHA 11. Shri J.P. Agarwal 12. Shri G.S. Basavaraj 13. Shri Kuldeep Bishnoi 14. Shri C.M. Chang 15. Shri Jayant Chaudhary 16. Shri K.P. Dhanapalan 17. Shri Shivaram Gouda 18. Shri Sk. Saidul Haque 19. Shri S. R. Jeyadurai 20. Shri Nalin Kumar Kateel 21. Shrimati Putul Kumari 22. Shri P. Lingam 23. Shri Baijayant ‘Jay’ Panda 24. Shri Kadir Rana 25. Shri Nama Nageswara Rao 26. Shri Vishnu Dev Sai 27. -

Union Cabinet Minister, India.Pdf
India gk World gk Misc Q&A English IT Current Affairs TIH Uninon Cabinet Minister of India Sl No Portfolio Name Cabinet Minister 1 Prime Minister Minister of Atomic Energy Minister of Space Manmohan Singh Minister of Personnel, Public Grievances and Pensions Ministry of Planning 2 Minister of Finance P. Chidambaram 3 Minister of External Affairs Salman Khurshid 4 Minister of Home Affairs Sushil Kumar Shinde 5 Minister of Defence A. K. Antony 6 Minister of Agriculture Sharad Pawar Minister of Food Processing Industries 7 Minister of Communications and Information Technology Kapil Sibal Minister of Law and Justice 8 Minister of Human Resource Development Dr. Pallam Raju 9 Ministry of Mines Dinsha J. Patel 10 Minister of Civil Aviation Ajit Singh 11 Minister of Commerce and Industry Anand Sharma Minister of Textiles 12 Minister of Petroleum and Natural Gas Veerappa Moily 13 Minister of Rural Development Jairam Ramesh 14 Minister of Culture Chandresh Kumari Katoch 15 Minister of Housing and Urban Poverty Alleviation Ajay Maken 16 Minister of Water Resources Harish Rawat 17 Minister of Urban Development Kamal Nath Minister of Parliamentary Affairs 18 Minister of Overseas Indian Affairs Vayalar Ravi 19 Minister of Health and Family Welfare Ghulam Nabi Azad 20 Minister of Labour and Employment Mallikarjun Kharge 21 Minister of Road Transport and Highways Dr. C. P. Joshi Minister of Railway 22 Minister of Heavy Industries and Public Enterprises Praful Manoharbhai Patel 23 Minister of New and Renewable Energy Farooq Abdullah 24 Minister of Panchayati Raj Kishore Chandra Deo Minister of Tribal Affairs 25 Minister of Science and Technology Jaipal Reddy Minister of Earth Sciences 26 Ministry of Coal Prakash Jaiswal 27 Minister of Steel Beni Prasad Verma 28 Minister of Shipping G. -

The Journal of Parliamentary Information
The Journal of Parliamentary Information VOLUME LVII NO. 2 JUNE 2011 LOK SABHA SECRETARIAT NEW DELHI CBS Publishers & Distributors Pvt. Ltd. 24, Ansari Road, Darya Ganj, New Delhi-2 2009 issue, EDITORIAL BOARD Editor : T.K. Viswanathan Secretary-General Lok Sabha Associate Editor : P.K. Misra Joint Secretary Lok Sabha Secretariat Kalpana Sharma Director Lok Sabha Secretariat Assistant Editors : Pulin B. Bhutia Joint Director Lok Sabha Secretariat Sanjeev Sachdeva Joint Director Lok Sabha Secretariat © Lok Sabha Secretariat, New Delhi for approval. THE JOURNAL OF PARLIAMENTARY INFORMATION VOLUME LVII NO. 2 JUNE 2011 CONTENTS PAGE EDITORIAL NOTE 101 ADDRESSES Address by the President to Parliament, 21 February 2011 103 ARTICLE Parliamentary Oversight of Human Rights: A Case Study of Disability in India—Deepali Mathur 116 PARLIAMENTARY EVENTS AND ACTIVITIES Conferences and Symposia 123 Birth Anniversaries of National Leaders 124 Exchange of Parliamentary Delegations 125 Bureau of Parliamentary Studies and Training 127 PRIVILEGE ISSUES 129 PROCEDURAL MATTERS 131 PARLIAMENTARY AND CONSTITUTIONAL DEVELOPMENTS 135 DOCUMENTS OF CONSTITUTIONAL AND PARLIAMENTARY INTEREST 143 SESSIONAL REVIEW Lok Sabha 151 Rajya Sabha 184 State Legislatures 205 RECENT LITERATURE OF PARLIAMENTARY INTEREST 210 APPENDICES I. Statement showing the work transacted during the Seventh Session of the Fifteenth Lok Sabha 219 (iv) II. Statement showing the work transacted during the Two Hundred and Twenty-Second Session of the Rajya Sabha 223 III. Statement showing the activities of the Legislatures of the States and Union Territories during the period 1 January to 31 March 2011 228 IV. List of Bills passed by the Houses of Parliament and assented to by the President during the period 1 January to 31 March 2011 235 V. -

Agriculture on Environment: Lok Sabha 2012-13
Agriculture on Environment: Lok Sabha 2012-13 Q. No. Q. Type Date Ans by Ministry Members Title of the Questions Subject Specific Political State Party Repres entative *11 Starred 13.03.2012 Agriculture Shri Narahari Use of Chemical Fertilisers Agriculture West Mahato AIFB Bengal Environmental Education, NGOs and Media Health and Sanitation Pollution *16 Starred 13.03.2012 Agriculture Shri Rajaiah Promotion of Animal Agriculture Andhra Siricilla Husbandry INC Pradesh Environmental Education, NGOs and Media 16 Unstarred 13.03.2012 Agriculture Shri Kaushalendra Misbranded Pesticides Agriculture Bihar Kumar JD(U) Shri Ramkishun Health and Sanitation Uttar SP Pradesh Pollution 19 Unstarred 13.03.2012 Agriculture Shri Badri Ram Barren Land Agriculture INC Rajasth Jakhar an Environmental Conservation 23 Unstarred 13.03.2012 Agriculture Shri Suresh Kumar Organic Farming Agriculture INC Andhra Shetkar Pradesh Environmental Conservation 37 Unstarred 13.03.2012 Agriculture Shri K. Sugumar Farm Technologies to Agriculture Tamil Farmers AIADM Nadu K Environmental Education, NGOs and Media 41 Unstarred 13.03.2012 Agriculture Shri Manicka Agreement with Israel in Agriculture Tamil Tagore Agriculture INC Nadu Environmental Education, NGOs and Media Forest Conservation 57 Unstarred 13.03.2012 Agriculture Shri Magunta Growth of Animal Agriculture INC Andhra Sreenivasulu Reddy Husbandry and Horticulture Pradesh Sectors Wildlife Management 71 Unstarred 13.03.2012 Agriculture Shri Rakesh Singh Structural Change in Agriculture Madhya Agriculture BJP Pradesh -

Title: Need to Check the Spread of Mysterious Viral Disease in Jalgaon District of Maharashtra. SHRI HARIBHAU JAWALE (RAVER): A
> Title: Need to check the spread of mysterious viral disease in Jalgaon district of Maharashtra. SHRI HARIBHAU JAWALE (RAVER): A mysterious viral disease has affected over 700 students in the age group of 10 to 17 years at school level in Northern Maharashtra specifically in Jalgaon district. For the last 15 days schools have declared holidays. The Students have complained of vomiting, headache, suffocation and giddiness. This was observed when some of the students during school's prayer, suddenly collapsed and started complaining. Due to lack of proper facilities at the Primary Health Centre and Gramin Hospitals of the villages, the students were brought to the nearby town hospitals. The symptoms of illness observed were sudden collapse with heavy vomitting sensation, highly suffocating with high fever. The parents of the students are in the frightened state and not sending their children to school. All the school education in the district had been hampered. The National Institute of Virology, Pune has given NIL Report by examining the samples taken from the affected students of different schools. The Government Health Department should work had for the detection of so called mysterious Viral Infections, might be spreading through air. I through this august House request the Government to please detect the problems and take necessary steps urgently to trace & locate the cause for this Viral disease, as the infection is spreading very rapidly in the area of Northern Maharashtra and has now started in Marathwada. . -

Committees of Rajya Sabha and Other Parliamentary Committees and Bodies on Which Rajya Sabha Is Represented (2018-19)
COMMITTEES OF RAJYA SABHA AND OTHER PARLIAMENTARY COMMITTEES AND BODIES ON WHICH RAJYA SABHA IS REPRESENTED (2018-19) (As on 7TH FEBRUARY, 2019) Com. Co-ord. Sec. PARLIAMENT OF INDIA R A J Y A S A B H A COMMITTEES OF RAJYA SABHA AND OTHER PARLIAMENTARY COMMITTEES AND BODIES ON WHICH RAJYA SABHA IS REPRESENTED (Corrected upto 7th February, 2019) RAJYA SABHA SECRETARIAT NEW DELHI (7TH FEBRUARY 2019) OFFICERS OF RAJYA SABHA CHAIRMAN Shri M. Venkaiah Naidu DEPUTY CHAIRMAN Shri Harivansh SECRETARY-GENERAL Shri Desh Deepak Verma PREFACE The publication aims at providing information on Members of Rajya Sabha serving on various Committees of Rajya Sabha, Department-related Parliamentary Standing Committees, Joint Committees and other Bodies. The names of Chairmen of the various Standing Committees and Department-related Parliamentary Standing Committees along with their local residential addresses and telephone numbers have also been shown at the beginning of the publication. The names of Members of the Lok Sabha serving on the Joint Committees on which Rajya Sabha is represented have also been included under the respective Committees for information. Change of nominations/elections of Members of Rajya Sabha in various Parliamentary Committees/Statutory Bodies is an ongoing process. As such, some information contained in the publication may undergo change by the time this is brought out. When new nominations/elections of Members to Committees/Statutory Bodies are made or changes in these take place, the same get updated in the Rajya Sabha website. The main purpose of this publication, however, is to serve as a primary source of information on Members representing various Committees and other Bodies on which Rajya Sabha is represented upto a particular period.