Can the Invasive Earthworm, Amynthas Agrestis, Be Controlled with Prescribed fire?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Three New Earthworm Species of the Genus Amynthas (Family Megascolecidae) from Northern Mountainous Part of Okinawa- Jima Island, Japan
Edaphologia, No. 103: 25–32, November 30, 2018 25 Three new earthworm species of the genus Amynthas (family Megascolecidae) from northern mountainous part of Okinawa- jima Island, Japan Yasufumi Azama1 and Kotaro Ishizuka2 13-12-55 Okita, Nago City, Okinawa, 905-0019 Japan 22-7-15 Kanayama-Cho, Higashi-kurume City, Tokyo, 203-0003 Japan Corresponding author: Yasufumi Azama ([email protected]) Received: 31 October 2017; Accepted 20 August 2018 Abstract Three new earthworm species of the genus Amynthas (Family Megascolecidae) were described from the forest area, northern part of Okinawa-jima Island. A. surculatus sp. nov. is characterized by single large circular sucker-shaped genital markings at mid-ventral line and post-setal on segments 7–8 (occasionally 6–8); scientific name refers to the large circular sucker-shaped genital marking at pre-clitellar. A. glaucus sp. nov. is characterized by thick- short figure and greenish body color; scientific name refers to greenish body color. A. cucurbitae sp. nov. is character- ized by large gourd-shaped colored patch types external markings, close to male pore; scientific name refers to the gourd-shaped external markings. Three new species are distinguishable from the other congeneric species by combina- tions of the following character status; 1) number of spermathecal pore, 2) number and situation of genital markings, 3) external markings, 4) spermathecae, 5) genital glands, 6) body size and body color. Key words: Amynthas, Megascolecidae, new species, Okinawa-jima Island keeping alive and anesthetized in water by dropping 70% Introduction ethanol. Anesthetized worms were killed by 10% formalin and To date, sixteen species of the genus Pheretima s. -

COMPETITIVE EXCLUSION, FORAGING INTERFERENCE, and HABITAT DEGRADATION REDUCE SALAMANDER DENSITY Julie L
John Carroll University Carroll Collected Masters Theses Theses, Essays, and Senior Honors Projects Summer 2015 INVASIVE ASIAN EARTHWORMS NEGATIVELY IMPACT WOODLAND SALAMANDERS: COMPETITIVE EXCLUSION, FORAGING INTERFERENCE, AND HABITAT DEGRADATION REDUCE SALAMANDER DENSITY Julie L. Ziemba John Carroll University, [email protected] Follow this and additional works at: http://collected.jcu.edu/masterstheses Part of the Biology Commons Recommended Citation Ziemba, Julie L., "INVASIVE ASIAN EARTHWORMS NEGATIVELY IMPACT WOODLAND SALAMANDERS: COMPETITIVE EXCLUSION, FORAGING INTERFERENCE, AND HABITAT DEGRADATION REDUCE SALAMANDER DENSITY" (2015). Masters Theses. 13. http://collected.jcu.edu/masterstheses/13 This Thesis is brought to you for free and open access by the Theses, Essays, and Senior Honors Projects at Carroll Collected. It has been accepted for inclusion in Masters Theses by an authorized administrator of Carroll Collected. For more information, please contact [email protected]. INVASIVE ASIAN EARTHWORMS NEGATIVELY IMPACT WOODLAND SALAMANDERS: COMPETITIVE EXCLUSION, FORAGING INTERFERENCE, AND HABITAT DEGRADATION REDUCE SALAMANDER DENSITY A Thesis Submitted to the Office of Graduate Studies College of Arts & Sciences of John Carroll University in Partial Fulfillment of the Requirements for the Degree of Master of Science By Julie L. Ziemba 2015 Table of Contents Abstract ..........................................................................................................................1 1. Introduction ................................................................................................................3 -

A Case Study of the Exotic Peregrine Earthworm Morphospecies Pontoscolex Corethrurus Shabnam Taheri, Céline Pelosi, Lise Dupont
Harmful or useful? A case study of the exotic peregrine earthworm morphospecies Pontoscolex corethrurus Shabnam Taheri, Céline Pelosi, Lise Dupont To cite this version: Shabnam Taheri, Céline Pelosi, Lise Dupont. Harmful or useful? A case study of the exotic peregrine earthworm morphospecies Pontoscolex corethrurus. Soil Biology and Biochemistry, Elsevier, 2018, 116, pp.277-289. 10.1016/j.soilbio.2017.10.030. hal-01628085 HAL Id: hal-01628085 https://hal.archives-ouvertes.fr/hal-01628085 Submitted on 5 Jan 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Harmful or useful? A case study of the exotic peregrine earthworm MARK morphospecies Pontoscolex corethrurus ∗ ∗∗ S. Taheria, , C. Pelosib, L. Duponta, a Université Paris Est Créteil, Université Pierre et Marie Curie, CNRS, INRA, IRD, Université Paris-Diderot, Institut d’écologie et des Sciences de l'environnement de Paris (iEES-Paris), Créteil, France b UMR ECOSYS, INRA, AgroParisTech, Université Paris-Saclay, 78026 Versailles, France ABSTRACT Exotic peregrine earthworms are often considered to cause environmental harm and to have a negative impact on native species, but, as ecosystem engineers, they enhance soil physical properties. Pontoscolex corethrurus is by far the most studied morphospecies and is also the most widespread in tropical areas. -

Checklist of Japanese Earthworms Updated from Easton (1981)
Checklist of Japanese Earthworms updated from Easton (1981) by R.J. Blakemore October, 2008 C/-, Soil Ecology Group, Yokohama National University, Japan. Summary The current list is about 82 valid species in seven families from Japan. Previous revisions by Easton (1981) had listed 73 or 74 species and Blakemore (2003) provisionally catalogued 76 valid earthworm taxa, with approximately 80 further names (ca. 50% of the total) either in synonymy or retained as species incertae sedis . Some 60 or so “new” Pheretima names with strange genus and species definition had been added by Ishizuka in 1999-2001 that, mostly ignorant of ICZN (1999), had but a few considered valid taxa with the majority being synonyms, homonyms [such as the permanently invalid primary homonym Pheretima montana 1999 (non Kinberg, 1867 – the type species of the genus)] or species incertae sedis and, as yet, no native Pheretima s. stricto are known from Japan. About 30 species are known introductions with four “new records” by the current author, and another ten are possibly more widespread (e.g. in Korea/China), thus the probable number of wholly endemic Japanese earthworms is around 40 species (ca. 50% of the total valid species). A subsequent report of Amynthas rodericensis (Grube, 1879) from Japan by a non-specialist is dubious and unconfirmed. However, a definitive work on the systematics of all of Japan’s earthworms is pending, relying partly on review of all of Japan’s and Korea’s classical species, and partly on more systematic on the ground eco-taxonomic surveys. While much of Easton’s synopsis is supported, Pontodrilus is now placed in Megascolecidae sensu Blakemore (2000) rather than Acanthodrilidae sensu Gates (1959); Amynthas carnosus (Goto & Hatai, 1899) is removed from synonymy of Amynthas gracilis ; and an informal Amynthas corticis species-complex is established to accommodate the various morphs of this widely distributed species group. -
Size Variation and Geographical Distribution of the Luminous Earthworm Pontodrilus Litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan
A peer-reviewed open-access journal ZooKeys 862: 23–43 (2019) Size variation and distribution of Pontodrilus litoralis 23 doi: 10.3897/zookeys.862.35727 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Size variation and geographical distribution of the luminous earthworm Pontodrilus litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan Teerapong Seesamut1,2,4, Parin Jirapatrasilp2, Ratmanee Chanabun3, Yuichi Oba4, Somsak Panha2 1 Biological Sciences Program, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand 2 Ani- mal Systematics Research Unit, Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand 3 Program in Animal Science, Faculty of Agriculture Technology, Sakon Nakhon Rajabhat University, Sakon Nakhon 47000, Thailand 4 Department of Environmental Biology, Chubu University, Kasugai 487-8501, Japan Corresponding authors: Somsak Panha ([email protected]), Yuichi Oba ([email protected]) Academic editor: Samuel James | Received 24 April 2019 | Accepted 13 June 2019 | Published 9 July 2019 http://zoobank.org/663444CA-70E2-4533-895A-BF0698461CDF Citation: Seesamut T, Jirapatrasilp P, Chanabun R, Oba Y, Panha S (2019) Size variation and geographical distribution of the luminous earthworm Pontodrilus litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan. ZooKeys 862: 23–42. https://doi.org/10.3897/zookeys.862.35727 Abstract The luminous earthworm Pontodrilus litoralis (Grube, 1855) occurs in a very wide range of subtropical and tropical coastal areas. Morphometrics on size variation (number of segments, body length and diameter) and genetic analysis using the mitochondrial cytochrome c oxidase subunit 1 (COI) gene sequence were conducted on 14 populations of P. -
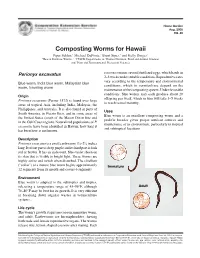
Composting Worms for Hawaii
Home Garden Aug. 2005 HG-46 Composting Worms for Hawaii Piper Selden,1 Michael DuPonte,2 Brent Sipes,3 and Kelly Dinges2 1Hawaii Rainbow Worms, 2, 3CTAHR Departments of 2Human Nutrition, Food and Animal Sciences and 3Plant and Environmental Protection Sciences Perionyx excavatus cocoon contains several fertilized eggs, which hatch in 2–3 weeks under suitable conditions. Reproductive rates Blue worm, India blue worm, Malaysian blue vary according to the temperature and environmental worm, traveling worm conditions, which in vermiculture depend on the maintenance of the composting system. Under favorable Origin conditions, blue worms may each produce about 20 offspring per week, which in turn will take 3–5 weeks Perionyx excavatus (Perrier 1872) is found over large to reach sexual maturity. areas of tropical Asia, including India, Malaysia, the Phillippines, and Australia. It is also found in parts of Uses South America, in Puerto Rico, and in some areas of Blue worm is an excellent composting worm and a the United States (south of the Mason Dixon line and prolific breeder given proper nutrient sources and in the Gulf Coast region). Naturalized populations of P. maintenance of its environment, particularly in tropical excavatus have been identified in Hawaii; how long it and subtropical locations. has been here is not known. Description 1 3 Perionyx excavatus is a small earthworm 1 ⁄4–2 ⁄4 inches long. Its front part is deep purple and its hind part is dark red or brown. It has an iridescent, blue-violet sheen on its skin that is visible in bright light. These worms are highly active and twitch when disturbed. -

The Giant Palouse Earthworm (Driloleirus Americanus)
PETITION TO LIST The Giant Palouse Earthworm (Driloleirus americanus) AS A THREATENED OR ENDANGERED SPECIES UNDER THE ENDANGERED SPECIES ACT June 30, 2009 Friends of the Clearwater Center for Biological Diversity Palouse Audubon Palouse Prairie Foundation Palouse Group of the Sierra Club 1 June 30, 2009 Ken Salazar, Secretary of the Interior Robyn Thorson, Regional Director U.S. Department of the Interior U.S. Fish & Wildlife Service 1849 C Street N.W. Pacific Region Washington, DC 20240 911 NE 11th Ave Portland, Oregon Dear Secretary Salazar, Friends of the Clearwater, Center for Biological Diversity, Palouse Prairie Foundation, Palouse Audubon, Palouse Group of the Sierra Club and Steve Paulson formally petition to list the Giant Palouse Earthworm (Driloleirus americanus) as a threatened or endangered species pursuant to the Endangered Species Act (”ESA”), 16 U.S.C. §1531 et seq. This petition is filed under 5 U.S.C. 553(e) and 50 CFR 424.14 (1990), which grant interested parties the right to petition for issuance of a rule from the Secretary of Interior. Petitioners also request that critical habitat be designated for the Giant Palouse Earthworm concurrent with the listing, pursuant to 50 CFR 424.12, and pursuant to the Administrative Procedures Act (5 U.S.C. 553). The Giant Palouse Earthworm (D. americanus) is found only in the Columbia River Drainages of eastern Washington and Northern Idaho. Only four positive collections of this species have been made within the last 110 years, despite the fact that the earthworm was historically considered “very abundant” (Smith 1897). The four collections include one between Moscow, Idaho and Pullman, Washington, one near Moscow Mountain, Idaho, one at a prairie remnant called Smoot Hill and a fourth specimen near Ellensberg, Washington (Fender and McKey- Fender, 1990, James 2000, Sánchez de León and Johnson-Maynard, 2008). -

Checklist of New Zealand Earthworms Updated from Lee (1959) by R.J
Checklist of New Zealand Earthworms updated from Lee (1959) by R.J. Blakemore August, 2006 COE fellow, Soil Ecology Group, Yokohama National Univeristy, Japan. Summary This review is based on Blakemore (2004) that updated the work completed over 40 years earlier by Lee (1959) as modified by Blakemore in Lee et al. , (2000) and in Glasby et al. (2007/8) based on the information presented at the "Species 2000" meeting held Jan. 2000 at Te Papa Museum in Wellington, New Zealand. In the current checklist Acanthodrilidae, Octochaetidae and Megascolecidae sensu Blakemore (2000b) are all given separate family status. Whereas Lee (1959) listed approximately 193 species, the current list has about 199 taxa. Because many of the natives have few reports, or are based on only a few specimens, approximately 77 are listed as "threatened" or "endangered" in Dept. Conservation threatened species list (see www.doc.govt.nz/Conservation/001~Plants-and-Animals/006~Threatened-species/Terrestrial-invertebrate-(part-one).asp April, 2005) and three species are detailed in McGuinness (2001). Further studies such as those of Springett & Grey (1998) are required. Currently I seek funding to complete my database into an interactive guide to species, to use to conduct surveys in New Zealand. Some of the changes in Blakemore (2004) from Lee (1959) are: • Microscolex macquariensis (Beddard, 1896) is removed from the list because it is known only from Macquarie Island, which is now claimed as Australian territory (see Blakemore, 2000b). • Megascolides orthostichon (Schmarda, 1861) is removed from the fauna as Fletcher (1886: 524) reported that “on the authority of Captain Hutton” this species was not from New Zealand and may be from Mt Wellington in Tasmania (see Blakemore, 2000b). -

Ecological Monitoring at Rare, 2020
Ecological Monitoring 2020 rare Charitable Research Reserve Prepared by: Jordan Wrobel Jenna Quinn 1 Acknowledgements Many thanks to Colleges and Institutes Canada (CICan) Career-Launcher Internships, funded by Natural Resource’s Canada’s Green Jobs- Science and Technology Internship Program, and Employment Ontario for providing essential funding for ecological monitoring at rare; without their support, this monitoring program and report would not have been possible. I would also like to thank rare staff for assistance with monitoring and support of intellectual and professional growth. Thank you to Caroline Reisiger and Sarah Cui for their much- appreciated assistance with fieldwork and to Dr. Justin Gaudon for your support with the statistical analyses. To rare’s committed volunteers: Jacqueline Haynes, Miriam Bauman, Emma Wegener, Hilary Irving, Bethany Wakefield, and Logan Mercier; thank you so much for your support with monitoring, these programs would not be as successful without you. I would like to thank all advocates of rare Charitable Research Reserve for helping to support rare’s vision and activities. The rare Charitable Research Reserve acknowledges and is grateful to all of the original stewards of the land in which rare resides, within the Haldimand Tract, spanning six miles on either side of the Grand River from source to mouth. Understanding that this land has been rich in diverse Indigenous presence since time immemorial, there are several Indigenous Nations that we would like to mention. We would like to honor and respect the sovereignty of both First Nations in our area: the Haudenosaunee Peoples of Six Nations of the Grand River and the Anishinaabe Peoples of Mississaugas of the New Credit First Nation. -

Biomolecular Approach to Oligochaete Taxonomy Dr
Dr. Jaya M et. al. / International Journal of New Technologies in Science and Engineering Vol. 2, Issue 6,Dec 2015, ISSN 2349-0780 Biomolecular Approach To Oligochaete Taxonomy Dr. Jaya. M 1* ,Dr. Aja. M2 and Dr. K. Vijayakumaran Nair3 1* Assistant Professor, Department of Zoology, Sree keralavarma College, Thrissur, Email: [email protected] 2Senior Research Fellow, Department of Zoology, University of Kerala, Kariavatom, Email: [email protected] 3Associate Professor, Department of Zoology, Mar Ivanios College, Thiruvananthapuram, Email: [email protected] ABSTRACT This paper comprises the molecular approach for the identification of earthworm along with the traditional taxonomic method. The mitochondrial CO 1 gene of the Pontoscolex corethrurus (Glossoscolecidae), Travoscolides chengannures, Amynthas corticis, Perionyx sansibaricus (Megascolecidae), Progizzardus varadiamensis and Glyphidrilus annandalei (Almidae) were sequenced. The cytochrome-c oxidase I (CO1) exhibited a unique barcode to a particular species. The further exploration of mitochondrial diversity in earthworms will lead to major improvements in our understanding of the evolutionary pathways and rates of the mitochondrial genome Key words: Barcoding, cytochrome c oxidase 1 (COI), 16S ribosomal DNA INTRODUCTION DNA barcoding is a taxonomic method that uses a short genetic marker in the DNA to identify an organism. It differs from molecular phylogeny in that the main goal is to identify an unknown sample in terms of a known classification [26]. DNA sequence can be used to identify different species, in the same way as the supermarket scanner uses the black stripes of the UPC barcode to identify the items. This database will rapidly link a specimen to a Binomial Linnaean name and through that link it will provide all available information and studies on the species. -

Amynthas) in the Northeast United States
Invertebrate Biology x(x): 1–14. © 2016 The Authors. Invertebrate Biology published by Wiley Periodicals, Inc. on behalf of American Microscopical Society. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made. DOI: 10.1111/ivb.12145 Phylogeographic analysis of invasive Asian earthworms (Amynthas) in the northeast United States Nancy Schult, Kelly Pittenger, Sam Davalos, and Damhnait McHugha Department of Biology, Colgate University, Hamilton, New York 13346, USA Abstract. Phylogeographic studies are useful in reconstructing the history of species inva- sions, and in some instances can elucidate cryptic diversity of invading taxa. This can help in predicting or managing the spread of invasive species. Among terrestrial invasive species in North America, earthworms can have profound ecological effects. We are familiar with the centuries-old invasions of European earthworms (Lumbricidae) and their impacts on nutrient cycling in soils. More recent invasions by Asian earthworms of the family Megascolecidae are less fully understood. We used data for two mitochondrial gene frag- ments, cytochrome oxidase I (COI) and 16S rRNA, to examine the relationships among populations of Asian earthworms in the megascolecid genus Amynthas in the northeast Uni- ted States. Recent reports have indicated that one species in particular, Amynthas agrestis, is having detrimental effects in mixed forest ecosystems, and we were interested in under- standing the invasion history for this species. We were surprised to discover three divergent mitochondrial lineages of Amynthas occurring sympatrically in upstate New York. -

Asian Jumping Worm (Megascolecidae) Impacts on Physical and Biological Characteristics of Turfgrass Ecosystems
Colby College Digital Commons @ Colby Honors Theses Student Research 2019 Asian Jumping Worm (Megascolecidae) Impacts on Physical and Biological Characteristics of Turfgrass Ecosystems Ella L. Maddi Colby College Follow this and additional works at: https://digitalcommons.colby.edu/honorstheses Part of the Environmental Sciences Commons, and the Soil Science Commons Colby College theses are protected by copyright. They may be viewed or downloaded from this site for the purposes of research and scholarship. Reproduction or distribution for commercial purposes is prohibited without written permission of the author. Recommended Citation Maddi, Ella L., "Asian Jumping Worm (Megascolecidae) Impacts on Physical and Biological Characteristics of Turfgrass Ecosystems" (2019). Honors Theses. Paper 965. https://digitalcommons.colby.edu/honorstheses/965 This Honors Thesis (Open Access) is brought to you for free and open access by the Student Research at Digital Commons @ Colby. It has been accepted for inclusion in Honors Theses by an authorized administrator of Digital Commons @ Colby. Asian Jumping Worm impacts (Megascolecidae) on Physical and Biological Characteristics of Turfgrass Ecosystems An Honors Thesis presented to the Faculty of the Department of Biology at Colby College in partial fulfillment of the requirements for the Degree of Bachelor of Arts with Honors By Ella Maddi Waterville, ME May 20, 2019 Asian Jumping Worm impacts (Megascolecidae) on Physical and Biological Characteristics of Turfgrass Ecosystems An Honors Thesis presented