Ventricular Septation and Outflow Tract Development in Crocodilians Result In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Caiman Crocodilus Fuscus
Facultad de Ciencias ACTA BIOLÓGICA COLOMBIANA Departamento de Biología http://www.revistas.unal.edu.co/index.php/actabiol Sede Bogotá ARTÍCULO DE INVESTIGACIÓN / RESEARCH ARTICLE ZOOLOGIA DETERMINATION OF HEMATOLOGICAL VALUES OF COMMON CROCODILE (Caiman crocodilus fuscus) IN CAPTIVITY IN THE MAGDALENA MEDIO OF COLOMBIA Determinación de valores hematológicos de caimán común (Caiman crocodilus fuscus) en cautiverio en el Magdalena Medio de Colombia Juana GRIJALBA O1 , Elkin FORERO1 , Angie CONTRERAS1 , Julio VARGAS1 , Roy ANDRADE1 1Facultad de Ciencias Agropecuarias, Universidad Pedagógica y Tecnológica de Colombia, Avenida Central del Norte 39-115, 150003 Tunja, Tunja, Boyacá, Colombia *For correspondence: [email protected] Received: 04th December 2018 , Returned for revision: 03rd March 2019, Accepted: 08th May 2019. Associate Editor: Nubia E. Matta. Citation/Citar este artículo como: Grijalba J, Forero E, Contreras A, Vargas J, Andrade R. Determination of hematological values of common crocodile (Caiman crocodilus fuscus) in captivity in the Magdalena Medio of Colombia. Acta biol. Colomb. 2020;25(1):75-81. DOI: http://dx.doi.org/10.15446/ abc.v25n1.76045 ABSTRACT Caiman zoo breeding (Caiman crocodilus fuscus) has been developing with greater force in Colombia since the 90s. It is essential to evaluate the physiological ranges of the species to be able to assess those situations in which their health is threatened. The objective of the present study was to determine the typical hematological values of the Caiman (Caiman crocodilus fuscus) with the aid of the microhematocrit, the cyanmethemoglobin technique, and a hematological analyzer. The blood samples were taken from 120 young animals of both sexes in good health apparently (males 44 and females 76). -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -

Congenital Abnormalities of the Aortic Arch: Revisiting the 1964 Stewart
Cardiovascular Pathology 39 (2019) 38–50 Contents lists available at ScienceDirect Cardiovascular Pathology Review Article Congenital abnormalities of the aortic arch: revisiting the 1964 ☆ Stewart classification Shengli Li a,⁎,HuaxuanWena,MeilingLianga,DandanLuoa, Yue Qin a,YimeiLiaoa, Shuyuan Ouyang b, Jingru Bi a, Xiaoxian Tian c, Errol R. Norwitz d,GuoyangLuoe,⁎⁎ a Department of Ultrasound, Shenzhen Maternity & Child Healthcare Hospital, Affiliated to Southern Medical University, Shenzhen, 518028, China b Department of Laboratory Medicine, Shenzhen Maternity & Child Healthcare Hospital, Affiliated to Southern Medical University, Shenzhen, 518028, China c Department of Ultrasound, Maternity & Child Healthcare Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, 538001, China d Department of Obstetrics & Gynecology, Tufts University School of Medicine, Boston, MA 02111 e Department of Obstetrics & Gynecology, Howard University, College of Medicine, Washington, DC 20060, USA article info abstract Article history: The traditional classification of congenital aortic arch abnormalities was described by James Stewart and col- Received 12 June 2018 leagues in 1964. Since that time, advances in diagnostic imaging technology have led to better delineation of Received in revised form 27 November 2018 the vasculature anatomy and the identification of previously unrecognized and unclassified anomalies. In this Accepted 28 November 2018 manuscript, we review the existing literature and propose a series of modifications to the original Stewart -

Vocalizations in Two Rare Crocodilian Species: a Comparative Analysis of Distress Calls of Tomistoma Schlegelii (Müller, 1838) and Gavialis Gangeticus (Gmelin, 1789)
NORTH-WESTERN JOURNAL OF ZOOLOGY 11 (1): 151-162 ©NwjZ, Oradea, Romania, 2015 Article No.: 141513 http://biozoojournals.ro/nwjz/index.html Vocalizations in two rare crocodilian species: A comparative analysis of distress calls of Tomistoma schlegelii (Müller, 1838) and Gavialis gangeticus (Gmelin, 1789) René BONKE1,*, Nikhil WHITAKER2, Dennis RÖDDER1 and Wolfgang BÖHME1 1. Herpetology Department, Zoologisches Forschungsmuseum Alexander Koenig (ZFMK), Adenauerallee 160, 53113 Bonn, Germany. 2. Madras Crocodile Bank Trust, P.O. Box 4, Mamallapuram, Tamil Nadu 603 104, S.India. *Corresponding author, R. Bonke, E-mail: [email protected] Received: 07. August 2013 / Accepted: 16. October 2014 / Available online: 17. January 2015 / Printed: June 2015 Abstract. We analysed 159 distress calls of five individuals of T. schlegelii for temporal parameters and ob- tained spectral parameters in 137 of these calls. Analyses of G. gangeticus were based on 39 distress calls of three individuals, of which all could be analysed for temporal and spectral parameters. Our results document differences in the call structure of both species. Distress calls of T. schlegelii show numerous harmonics, whereas extensive pulse trains are present in G. gangeticus. In the latter, longer call durations and longer in- tervals between calls resulted in lower call repetition rates. Dominant frequencies of T. schlegelii are higher than in G. gangeticus. T. schlegelii specimens showed a negative correlation of increasing body size with de- creasing dominant frequencies. Distress call durations increased with body size. T. schlegelii distress calls share only minor structural features with distress calls of G. gangeticus. Key words: Tomistoma schlegelii, Gavialis gangeticus, distress calls, temporal parameters, spectral parameters. -

Development of the Vascular System in Five to Twenty-One
THE DEVELOPMENT OF THE VASCULAR SYSTEM IN FIVE TO TWtNTY-ONE SOMITE DOG EMBRYOS by ELDEN WILLIAM MARTIN B, S., Kansas State College of Agriculture and ADolied Science, 195>U A THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Zoology KANSAS STATV: COLLEGE OF AGRICULTURE AND A PLIED SCIENCE 1958 LP TH Ooco/*>*Tv TABLE OF CONTENTS INTRO IXJ CTION AND LITERATURE REVIEW 1 MATERIALS AND METHODS ^ OBSERVATIONS 6 Five-Somi te Stag© . 6 Seven-Somite Stage 8 Eight-Somite Stage 9 Ten- and bleven-Somite Stage 12 Twe 1 ve-Somi te Stage • \\i Fifteen-Somite Stage 18 Seventeen-Somite Stage 21 Eighteen-Somite Stage 2$ Twenty- and Twenty- one -Somite Stage 27 INTERPRETATIONS AND DISCUSSION 30 Vasculogenesis • 30 Cardiogenesis 33 The Origin and Development of Arteries \ 3lj. Aortic Arches •••« 3I4. Cranial Arterie s ...•• 36 The Dorsal Aorta 37 Intersegmental AAteries 39 Vertebral Arteries 39 Vitelline Arteries }±q The Allantoic Artery \±\ Ill IITERPRETATION AND DISCUSSION (Contd.) The Origin and Development of Veins •• kl The Anterior Cardinal Veins . I4.I Posterior Cardinal Veins k2 Umbilical Veins U3 Common Cardinal Veins kh Interconnecting Vessels Ui> SUMMARY kl LITERA°URE CITED $1 ACKNOWLEDGMENTS 53 APPENDIX 5U HTmDUCTIOW AND LITFRATORF. rfvibw While the dog has been employed extensively as a labora- tory animal in various fields of scientific endeavour, the use of this animal in embryology has been neglected. As a con- sequence, the literature on the circulatory system of the dog was represented only by an unpublished thesis by Duffey (3) on oardlogenesis and the first heart movements. -

CAIMAN CARE Thomas H
CAIMAN CARE Thomas H. Boyer, DVM, DABVP, Reptile and Amphibian Practice 9888-F Carmel Mountain Road, San Diego, CA, 92129 858-484-3490 www.pethospitalpq.com – www.facebook.com/pethospitalpq The spectacled caiman (Caiman crocodilus) is a popular animal among reptile enthusiasts. It is easy to understand their appeal, hatchlings are widely available outside California and make truly fascinating pets. Unfortuneately, if fed and housed properly they can grow a foot per year for the first few years and can rapidly outgrow their accommodations. Crocodilians are illegal in California without special permits. Most crocodilians are severely endangered (some are close to extinction) but spectacled caimans are one of the few species that aren't, therefore zoos are not interested in keeping them. Within a few years the endearing pet becomes a problem that nobody, including the owner, wants. They are difficult to give away. Some elect euthanasia at this point but most caimans die from inadequate care before they get big enough to become a problem. Other crocodilians are so severely endangered that it is illegal to own or trade in them, live or dead, without federal permits. Obviously I discourage individuals from purchasing an animal that within a few years will be an unsuitable pet. Although I can't endorse caimans as pets, I still feel if one has a caiman it should be cared for properly. One must realize that almost all crocodilians (the American Alligator is an exception) are tropical reptiles, thus they need a warm environment. Water temperature should be 75 to 80 F at all times. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

Phylogenetic Taphonomy: a Statistical and Phylogenetic
Drumheller and Brochu | 1 1 PHYLOGENETIC TAPHONOMY: A STATISTICAL AND PHYLOGENETIC 2 APPROACH FOR EXPLORING TAPHONOMIC PATTERNS IN THE FOSSIL 3 RECORD USING CROCODYLIANS 4 STEPHANIE K. DRUMHELLER1, CHRISTOPHER A. BROCHU2 5 1. Department of Earth and Planetary Sciences, The University of Tennessee, Knoxville, 6 Tennessee, 37996, U.S.A. 7 2. Department of Earth and Environmental Sciences, The University of Iowa, Iowa City, Iowa, 8 52242, U.S.A. 9 email: [email protected] 10 RRH: CROCODYLIAN BITE MARKS IN PHYLOGENETIC CONTEXT 11 LRH: DRUMHELLER AND BROCHU Drumheller and Brochu | 2 12 ABSTRACT 13 Actualistic observations form the basis of many taphonomic studies in paleontology. 14However, surveys limited by environment or taxon may not be applicable far beyond the bounds 15of the initial observations. Even when multiple studies exploring the potential variety within a 16taphonomic process exist, quantitative methods for comparing these datasets in order to identify 17larger scale patterns have been understudied. This research uses modern bite marks collected 18from 21 of the 23 generally recognized species of extant Crocodylia to explore statistical and 19phylogenetic methods of synthesizing taphonomic datasets. Bite marks were identified, and 20specimens were then coded for presence or absence of different mark morphotypes. Attempts to 21find statistical correlation between trace types, marking animal vital statistics, and sample 22collection protocol were unsuccessful. Mapping bite mark character states on a eusuchian 23phylogeny successfully predicted the presence of known diagnostic, bisected marks in extinct 24taxa. Predictions for clades that may have created multiple subscores, striated marks, and 25extensive crushing were also generated. Inclusion of fossil bite marks which have been positively 26associated with extinct species allow this method to be projected beyond the crown group. -

6 Development of the Great Vessels and Conduction Tissue
Development of the Great Vessels and Conduc6on Tissue Development of the heart fields • h:p://php.med.unsw.edu.au/embryology/ index.php?6tle=Advanced_-_Heart_Fields ! 2 Septa6on of the Bulbus Cordis Bulbus Cordis AV Canal Ventricle Looking at a sagital sec6on of the heart early in development the bulbus cordis is con6nuous with the ventricle which is con6nuous with the atria. As the AV canal shiOs to the right the bulbus move to the right as well. Septa6on of the Bulbus Cordis A P A P The next three slides make the point via cross sec6ons that the aorta and pulmonary arteries rotate around each other. This means the septum between them changes posi6on from superior to inferior as well. Septa6on of the Bulbus Cordis P A A P Septa6on of the Bulbus Cordis P A P A Migra6on of neural crest cells Neural crest cells migrate from the 3ed, 4th and 6th pharyngeal arches to form some of the popula6on of cells forming the aor6copulmonary septum. Septa6on of the Bulbus Cordis Truncal (Conal) Swellings Bulbus Cordis The cardiac jelly in the region of the truncus and conus adds the neural crest cells and expands as truncal swellings. Septa6on of the Bulbus Cordis Aorticopulmonary septum These swellings grow toward each other to meet and form the septum between the aorta and pulmonary artery. Aorta Pulmonary Artery Septa6on of the Bulbus Cordis Anterior 1 2 3 1 2 3 The aor6copulmonary septum then rotates as it moves inferiorly. However, the exact mechanism for that rota6on remains unclear. Septa6on of the Bulbus Cordis Aorta Pulmonary Artery Conal Ridges IV Foramen Membranous Muscular IV Endocarial Septum Interventricular Cushion Septum However, the aor6copulmonary septum must form properly for the IV septum to be completed. -

Human Placenta Is a Potent Hematopoietic Niche Containing Hematopoietic Stem and Progenitor Cells Throughout Development
Cell Stem Cell Article Human Placenta Is a Potent Hematopoietic Niche Containing Hematopoietic Stem and Progenitor Cells throughout Development Catherine Robin,1,5 Karine Bollerot,1,5 Sandra Mendes,1 Esther Haak,1 Mihaela Crisan,1 Francesco Cerisoli,1 Ivoune Lauw,1 Polynikis Kaimakis,1 Ruud Jorna,1 Mark Vermeulen,3 Manfred Kayser,3 Reinier van der Linden,1 Parisa Imanirad,1 Monique Verstegen,2 Humaira Nawaz-Yousaf,1 Natalie Papazian,2 Eric Steegers,4 Tom Cupedo,2 and Elaine Dzierzak1,* 1Erasmus MC Stem Cell Institute, Department of Cell Biology 2Department of Hematology 3Department of Forensic Molecular Biology 4Department of Obstetrics and Gynecology Erasmus University Medical Center, 3000 CA Rotterdam, the Netherlands 5These authors contributed equally to this work *Correspondence: [email protected] DOI 10.1016/j.stem.2009.08.020 SUMMARY becomes hematopoietic. The emergence of multipotent progen- itors and HSCs, organized in clusters of cells closely adherent to Hematopoietic stem cells (HSCs) are responsible for the ventral wall of the dorsal aorta, starts at day 27 in the devel- the life-long production of the blood system and are oping splanchnopleura/AGM region (Tavian et al., 1996, 1999, pivotal cells in hematologic transplantation thera- 2001). Starting at day 30, the first erythroid progenitors (BFU- pies. During mouse and human development, the E, burst forming unit erythroid) are found in the liver, with multi- first HSCs are produced in the aorta-gonad-meso- lineage hematopoietic progenitors (CFU-Mix or -GEMM; colony nephros region. Subsequent to this emergence, forming unit granulocyte, erythroid, macrophage, megakaryo- cyte) appearing in this tissue at week 13 (Hann et al., 1983). -
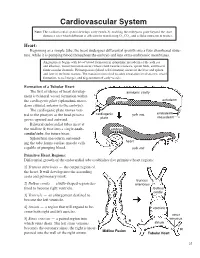
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

Aortic Arches
Human Embryology: Heart Development II Kimara L. Targoff, M.D. Division of Pediatric Cardiology, Columbia University Medical Center Developmental Genetics Program, Skirball Institute, NYU School of Medicine Human Vascular Development • Overview • Aortic Arch Development • Arterial Vascular Development • Venous System Development • Lymphatic Development • Transition from Fetal to Post-Natal Circulation Development of the Arterial and Venous Systems Cranial Ends of the Dorsal Aortae Form a Dorsoventral Loop: The First Aortic Arch Aortic Arches Arise in a Craniocaudal Sequence Surrounding the Pharynx Aortic Arches Give Rise to Important Head, Neck, and Upper Thorax Vessels Aortic Arch Development in the Chick Embryo Fgf8 is Required for Pharyngeal Arch Development in Mouse Abu-Issa, R. et al., Development 2002. Cardiovascular and Thymic Defects in Tbx1 Hypomorphic Mutant Neonates Hu, T. et al., Development 2004. Aortic Arch Development Dorsal aorta 1 2 3 Ventral aorta 4 5 6 7 iseg Harsh Thaker Aortic Arch Development Dorsal aorta 1 2 3 Ventral aorta 4 5 6 7 iseg Harsh Thaker Aortic Arch and Derivatives 3 3 4 4 7 iseg 6 7 iseg 6 Aortic sac Truncus arteriosus Harsh Thaker Aortic Arch and Derivatives 3 3 4 4 7 iseg 6 7 iseg Harsh Thaker Aortic Arch and Derivatives 3 3 4 7 iseg 4 7 iseg 6 Harsh Thaker Aortic Arch and Derivatives RCC LCC RSC LSC BCA DA Harsh Thaker Recurrent Laryngeal Nerves RCC LCC RSC LSC BCA DA Harsh Thaker Defects in Normal Regression of the Arterial System Lead to Vascular Anomalies • Double Aortic Arch – Failure of the