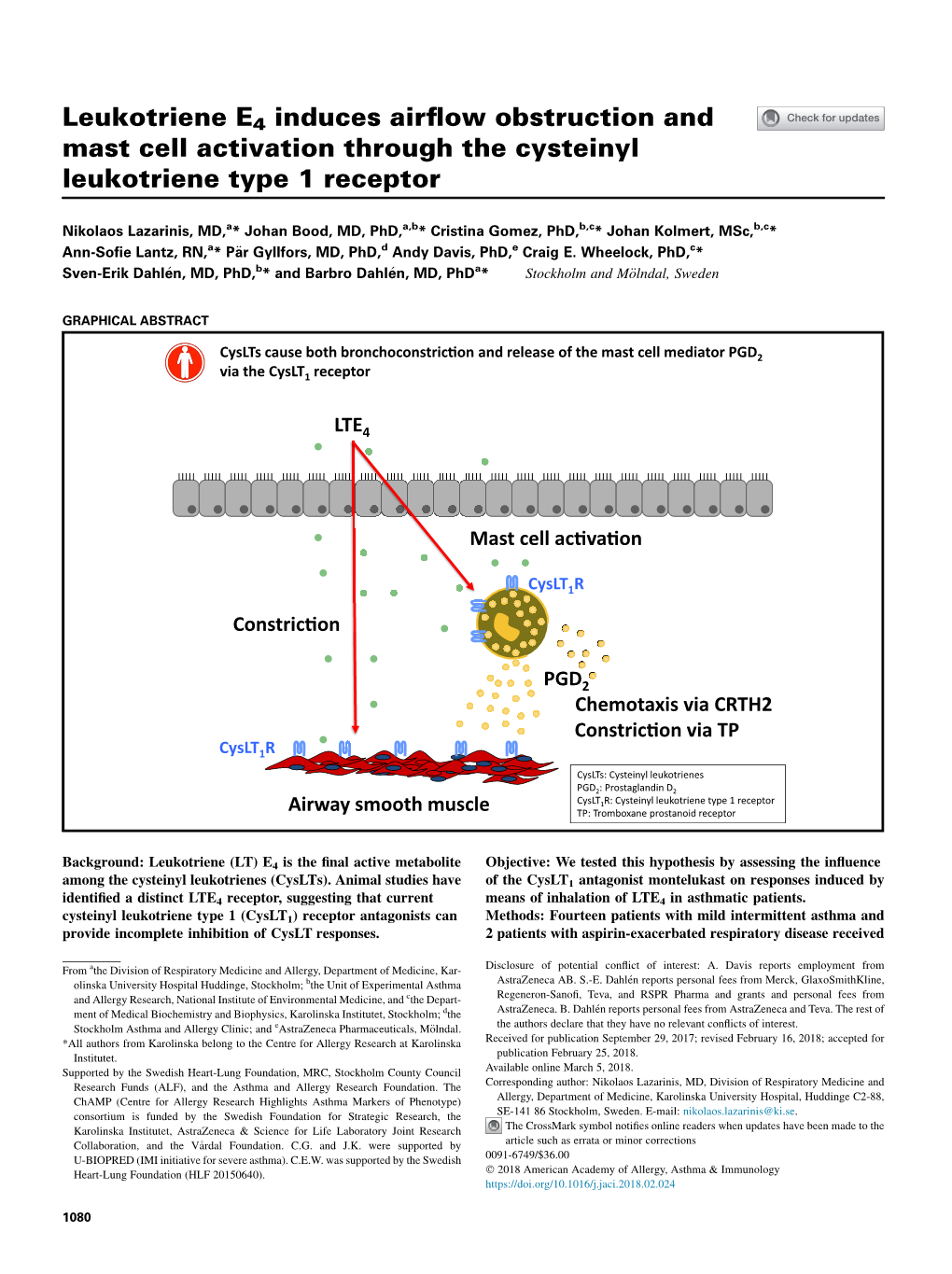
Leukotriene E4 Induces Airflow Obstruction and Mast Cell Activation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Montelukast, a Leukotriene Receptor Antagonist, Reduces the Concentration of Leukotrienes in the Respiratory Tract of Children with Persistent Asthma
Montelukast, a leukotriene receptor antagonist, reduces the concentration of leukotrienes in the respiratory tract of children with persistent asthma Benjamin Volovitz, MD,a,b Elvan Tabachnik, MD,c Moshe Nussinovitch, MD,b Biana Shtaif, MSc,b Hanna Blau, MD,a Irit Gil-Ad, PhD,b Abraham Weizman, MD,b and Itzhak Varsano, MDa,b Petah Tikva, Tel Aviv, and Rehovot, Israel Background: Leukotrienes are bronchoactive mediators secreted by inflammatory cells in the respiratory mucosa on Abbreviations used exposure to asthma triggers. BAL: Bronchoalveolar lavage Objective: We investigated the effect of montelukast, a CysLT1: Cysteinyl leukotriene 1 (receptor) leukotriene receptor antagonist, on the release of leukotrienes ECP: Eosinophilic cationic protein in the respiratory mucosa of children with persistent asthma. LTC4: Leukotriene C4 Method: Twenty-three children aged 6 to 11 years with moder- LTD4: Leukotriene D4 ately severe asthma were treated in a cross-over design start- LTE4: Leukotriene E4 ing, after a 2-week run in period, with either montelukast (n = 12) or cromolyn (n = 11) for 4 weeks with a 2-week washout period between treatments. Twelve of them were then treated Cysteinyl leukotrienes are potent proinflammatory with either montelukast or beclomethasone for 6 months. The mediators produced from a variety of inflammatory use of β -agonists was recorded on a diary card. The concen- 2 cells, including mast cells, eosinophils, basophils and tration of leukotriene C4 (LTC4) was measured by HPLC in nasal washes obtained before and at the end of each treatment macrophages. Leukotriene C4 (LTC4) is metabolized period. Eosinophilic cationic protein (ECP) was measured in enzymatically to leukotriene D4 (LTD4) and subsequent- the nasal washes by RIA. -

LEUKOTRIENE A4 HYDROLASE Martin J. Mueller
Department of Medical Biochemistry and Biophysics, Division of Chemistry II, Karolinska Institutet, 171 77 Stockholm, SWEDEN LEUKOTRIENE A4 HYDROLASE Identification of amino acid residues involved in catalyses and substrate-mediated inactivation Martin J. Mueller Stockholm 2001 Published and printed by Karolinska University Press Box 200, SE-171 77 Stockholm, Sweden © Martin J. Mueller, 2001 ISBN 91-628-4934-4 Abstract Leukotriene (LT) A4 hydrolase catalyzes the committed step in the biosynthesis of LTB4, a classical chemoattractant and immune-modulating lipid mediator involved in inflammation, host-defense against infections, and systemic, PAF-mediated, lethal shock. LTA4 hydrolase is a bifunctional zinc metalloenzyme with a chloride-stimulated arginyl aminopeptidase activity. When exposed to its lipid substrate LTA4, the enzyme is inactivated and covalently modified in a process termed suicide inactivation, which puts a restrain on the enzyme's ability to form the biologically active LTB4. In the present thesis, chemical modification with a series of amino acid-specific reagents, in the presence and absence of competitive inhibitors, was used to identify catalytically important residues at the active site. Thus, using differential labeling techniques, modification with the tyrosyl reagents N-acetylimidazole and tetranitromethane revealed the presence of two catalytically important Tyr residues. Likewise, modification with 2,3-butanedione and phenylglyoxal indicated that three Arg residues were located at, or near, the active center of the enzyme. Using differential Lys-specific peptide mapping of untreated and suicide inactivated LTA4 hy- drolase, a 21 residue peptide termed K21, was identified that is involved in binding of LTA4 to the native protein. Isolation and amino acid sequencing of a modified form of K21, revealed that Tyr- 378 is the site of attachment between LTA4 and the protein. -

Unlocking the Non-Ige-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2)
cells Review Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2) Mukesh Kumar, Karthi Duraisamy and Billy-Kwok-Chong Chow * School of Biological Sciences, The University of Hong Kong, Pokfulam Road, Hong Kong, China; [email protected] (M.K.); [email protected] (K.D.) * Correspondence: [email protected]; Tel.: +852-2299-0850; Fax: +852-2559-9114 Abstract: Mas-related G-protein coupled receptor member X2 (MRGPRX2) is a class A GPCR ex- pressed on mast cells. Mast cells are granulated tissue-resident cells known for host cell response, allergic response, and vascular homeostasis. Immunoglobulin E receptor (Fc"RI)-mediated mast cell activation is a well-studied and recognized mechanism of allergy and hypersensitivity reac- tions. However, non-IgE-mediated mast cell activation is less explored and is not well recognized. After decades of uncertainty, MRGPRX2 was discovered as the receptor responsible for non-IgE- mediated mast cells activation. The puzzle of non-IgE-mediated pseudo-allergic reaction is unlocked by MRGPRX2, evidenced by a plethora of reported endogenous and exogenous MRGPRX2 ag- onists. MRGPRX2 is exclusively expressed on mast cells and exhibits varying affinity for many molecules such as antimicrobial host defense peptides, neuropeptides, and even US Food and Drug Administration-approved drugs. The discovery of MRGPRX2 has changed our understanding of mast cell biology and filled the missing link of the underlying mechanism of drug-induced MC degranulation and pseudo-allergic reactions. These non-canonical characteristics render MRGPRX2 Citation: Kumar, M.; Duraisamy, K.; Chow, B.-K.-C. -

Inflammation, Cancer and Oxidative Lipoxygenase Activity Are Intimately Linked
Cancers 2014, 6, 1500-1521; doi:10.3390/cancers6031500 OPEN ACCESS cancers ISSN 2072-6694 www.mdpi.com/journal/cancers Review Inflammation, Cancer and Oxidative Lipoxygenase Activity are Intimately Linked Rosalina Wisastra and Frank J. Dekker * Pharmaceutical Gene Modulation, Groningen Research Institute of Pharmacy, University of Groningen, Antonius Deusinglaan 1, 9713 AV Groningen, The Netherlands; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +31-5-3638030; Fax: +31-5-3637953. Received: 16 April 2014; in revised form: 27 June 2014 / Accepted: 2 July 2014 / Published: 17 July 2014 Abstract: Cancer and inflammation are intimately linked due to specific oxidative processes in the tumor microenvironment. Lipoxygenases are a versatile class of oxidative enzymes involved in arachidonic acid metabolism. An increasing number of arachidonic acid metabolites is being discovered and apart from their classically recognized pro-inflammatory effects, anti-inflammatory effects are also being described in recent years. Interestingly, these lipid mediators are involved in activation of pro-inflammatory signal transduction pathways such as the nuclear factor κB (NF-κB) pathway, which illustrates the intimate link between lipid signaling and transcription factor activation. The identification of the role of arachidonic acid metabolites in several inflammatory diseases led to a significant drug discovery effort around arachidonic acid metabolizing enzymes. However, to date success in this area has been limited. This might be attributed to the lack of selectivity of the developed inhibitors and to a lack of detailed understanding of the functional roles of arachidonic acid metabolites in inflammatory responses and cancer. -

Levels of Prostaglandin E Metabolite And
Published OnlineFirst March 31, 2009; DOI: 10.1158/1940-6207.CAPR-09-0005 Published Online First on March 31, 2009 as 10.1158/1940-6207.CAPR-09-0005 Cancer Prevention Research Levels of Prostaglandin E Metabolite and Leukotriene E4 Are Increased in the Urine of Smokers: Evidence that Celecoxib Shunts Arachidonic Acid into the 5-Lipoxygenase Pathway Anna J. Duffield-Lillico,1,2 Jay O. Boyle,2 Xi Kathy Zhou,3 Aradhana Ghosh,4 Geera S. Butala,2 Kotha Subbaramaiah,4 Robert A. Newman,5 Jason D. Morrow,6 Ginger L. Milne6 and Andrew J. Dannenberg4 Abstract Cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LO) play a role in inflammation and car- cinogenesis. Biomarkers that reflect tobacco smoke–induced tissue injury are needed. In this study, levels of urinary prostaglandin E metabolite (PGE-M) and leukotriene E4 (LTE4), biomarkers of the COX and 5-LO pathways, were compared in never smokers, former smo- kers, and current smokers. The effects of celecoxib, a selective COX-2 inhibitor, on levels of PGE-M and LTE4 were determined. Baseline levels of PGE-M and LTE4 were positively as- sociated with smoking status; levels of PGE-M and LTE4 were higher in current versus never smokers. Treatment with 200 mg celecoxib twice daily for 6 ± 1 days led to a reduction in urinary PGE-M levels in all groups but exhibited the greatest effect among subjects with high baseline PGE-M levels. Thus, high baseline PGE-M levels in smokers reflected increased COX-2 activity. In individuals with high baseline PGE-M levels, treatment with celecoxib led to a significant increase in levels of urinary LTE4, an effect that was not found in indivi- duals with low baseline PGE-M levels. -

Signaling and Regulation of Cysteinyl Leukotriene Receptors in Intestinal Epithelial Cells and Colon Cancer Bengtsson, Astrid
Signaling and regulation of cysteinyl leukotriene receptors in intestinal epithelial cells and colon cancer Bengtsson, Astrid 2009 Link to publication Citation for published version (APA): Bengtsson, A. (2009). Signaling and regulation of cysteinyl leukotriene receptors in intestinal epithelial cells and colon cancer. Lund University. Total number of authors: 1 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. LUND UNIVERSITY PO Box 117 221 00 Lund +46 46-222 00 00 From the Department of Laboratory Medicine, Division of Cell Pathology, Lund University, Malmö, Sweden Signaling and regulation of cysteinyl leukotriene receptors in intestinal epithelial cells and colon cancer Astrid Bengtsson Academic dissertation By due permission of the Faculty of Medicine, Lund University, Sweden, to be publicly defended in the lecture hall, Clinical Research Center, Entrance 72, Malmö University Hospital (UMAS), Malmö on Friday, May 15, 2009, at 1 p.m. -

Increase in Urinary Leukotriene LTE4 Levels in Acute Asthma: Correlation with Airflow Limitation S a Green, M-P Malice, W Tanaka, C a Tozzi, T F Reiss
100 ASTHMA Thorax: first published as 10.1136/thorax.2003.006825 on 3 February 2004. Downloaded from Increase in urinary leukotriene LTE4 levels in acute asthma: correlation with airflow limitation S A Green, M-P Malice, W Tanaka, C A Tozzi, T F Reiss ............................................................................................................................... Thorax 2004;59:100–104. doi: 10.1136/thx.2004.006825 Background: Leukotrienes play a key role in the pathophysiology of chronic asthma. Activation of leukotriene pathways is accompanied by rises in detectable urinary levels of leukotriene E4 (LTE4). The relationship between urinary LTE4 levels and factors associated with acute asthma has not been determined. Methods: Adults aged 15–54 years presenting with moderate to severe acute asthma were evaluated at See end of article for emergency departments in 16 US sites. Forced expiratory volume in 1 second (FEV1) was measured authors’ affiliations during the first 60 minutes after arrival and at specified times until discharge or admission. Urine samples ....................... for measurement of LTE4 levels were obtained either on arrival at the study site and/or before discharge. Correspondence to: Patients were seen 2 weeks later for follow up, at which time repeat FEV1 measurements and urine samples Dr S A Green, Director, for LTE4 were obtained. Respiratory & Allergy, Results: One hundred and eighty four patients were evaluated; LTE4 results from both the acute and follow Merck Research up periods were available for analysis in 146. Urinary LTE levels were increased during asthma Laboratories, 126 East 4 Lincoln Avenue, RY34B- exacerbations compared with levels obtained 2 weeks later (geometric means 111.7 and 75.6 pg/mg 340, Rahway, NJ, USA; creatinine, respectively, mean percentage change 232.3; 95% confidence interval (CI) for the mean [email protected] percentage change 239.6 to 224.3, p,0.001). -

Concentration-Dependent Noncysteinyl Leukotriene Type 1 Receptor-Mediated Inhibitory Activity of Leukotriene Receptor Antagonist
Concentration-Dependent Noncysteinyl Leukotriene Type 1 Receptor-Mediated Inhibitory Activity of Leukotriene Receptor Antagonists This information is current as of September 27, 2021. Grzegorz Woszczek, Li-Yuan Chen, Sara Alsaaty, Sahrudaya Nagineni and James H. Shelhamer J Immunol 2010; 184:2219-2225; Prepublished online 18 January 2010; doi: 10.4049/jimmunol.0900071 Downloaded from http://www.jimmunol.org/content/184/4/2219 References This article cites 38 articles, 10 of which you can access for free at: http://www.jimmunol.org/ http://www.jimmunol.org/content/184/4/2219.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 27, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2010 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Concentration-Dependent Noncysteinyl Leukotriene Type 1 Receptor-Mediated Inhibitory Activity of Leukotriene Receptor Antagonists Grzegorz Woszczek,*,†,1 Li-Yuan Chen,*,1 Sara Alsaaty,* Sahrudaya Nagineni,* and James H. Shelhamer* The use of cysteinyl leukotriene receptor antagonists (LTRAs) for asthma therapy has been associated with a significant degree of interpatient variability in response to treatment. -

Botanical Drugs As an Emerging Strategy in Inflammatory
BOTANICAL DRUGS AS AN EMERGING STRATEGY IN INFLAMMATORY BOWEL DISEASE: A REVIEW Francesca Algieri, Alba Rodriguez-Nogales, M. Elena Rodriguez-Cabezas, Severiano Risco, M. Angeles Ocete*, Julio Galvez* CIBER-EHD, Department of Pharmacology, ibs.GRANADA, Center for Biomedical Research (CIBM), University of Granada, Granada, Spain Corresponding author: Julio Gálvez, Department of Pharmacology, Center for Biomedical Research, University of Granada, Avenida del Conocimiento s/n 18100- Armilla, Granada, Spain. E-mail: [email protected] Tel: 34-958-241793. Fax: 34-958- 248964. *Both authors contribute equally to the supervision of the study ABSTRACT Crohn’s disease and ulcerative colitis are the two most common categories of inflammatory bowel disease (IBD), which are characterized by chronic inflammation of the intestine that comprises the patients’ life quality and requires sustained pharmacological and surgical treatments. Since their aetiology is not completely understood, non-fully efficient drugs have been developed and those that show effectiveness are not devoid of quite important adverse effects that impair their long term use. Therefore, many patients try with some botanical drugs, which are related safe and efficient after many years of use. However, it is necessary to properly evaluate these therapies to consider a new strategy for human IBD. In this report we have reviewed the main botanical drugs that have been assesed in clinical trials in human IBD, and the mechanisms and the active compounds proposed for their beneficial effects. KEY WORDS: inflammatory bowel disease, botanical drugs, alteranative and complementary medicine, natural compounds, intestinal antiinflammatory activity INTRODUCTION Inflammatory bowel disease (IBD) is a chronic gastrointestinal inflammatory disorder characterized by alternating relapses and remissions. -

Thematic Review
thematic review Thematic Review Series: Proteomics An integrated omics analysis of eicosanoid biology1 Matthew W. Buczynski, Darren S. Dumlao, and Edward A. Dennis2 Department of Chemistry and Biochemistry, Department of Pharmacology, and School of Medicine, University of California, San Diego, La Jolla, CA 92093 Abstract Eicosanoids have been implicated in a vast number to address the question of how molecular biology works as of devastating inflammatory conditions, including arthritis, an integrated process (1). atherosclerosis, pain, and cancer. Currently, over a hundred Systems biology has advanced exponentially during the different eicosanoids have been identified, with many having past two decades, with transcriptomics, proteomics, and potent bioactive signaling capacity. These lipid metabolites metabolomics each playing an integral role. Each of these are synthesized de novo by at least 50 unique enzymes, many of which have been cloned and characterized. Due to the ex- platforms brings its own unique advantages and limitations tensive characterization of eicosanoid biosynthetic pathways, in facilitating the investigation of disease pathology. A this field provides a unique framework for integrating geno- transcriptomic approach can detect the upregulation and mics, proteomics, and metabolomics toward the investigation downregulation of important biosynthetic and signaling of disease pathology. To facilitate a concerted systems biol- genes; however, gene changes often donʼt directly corre- ogy approach, this review outlines the -

The Effects of Curcuma Domestica, Zingiber Officinale and Magnesium for Migraine Prophylaxis Brett R
Short Communication iMedPub Journals Journal of Nutraceuticals and Food Science 2016 http://www.imedpub.com/ Vol.1 No.1:3 The Effects of Curcuma domestica, Zingiber officinale and Magnesium for Migraine Prophylaxis Brett R. Martin DC Doctorate of Chiropractic National University of Health Sciences Pinellas Park, Florida, USA Corresponding author: Brett R. Martin, Doctorate of Chiropractic National University of Health Sciences Pinellas Park, Florida, USA, E-mail: [email protected] Received: January 19, 2016, Accepted: March 03, 2016, Published: March 05, 2016 Citation: Brett R. Martin DC, The Effects of Curcuma domestica, Zingiber officinale and Magnesium for Migraine Prophylaxis. 2016;1:1. to the intensity of the reflexive response, vasomotor tone Abstract cannot be reestablished in some cases for 24-48 hours [3]. There are several theories that have been employed to In the US and worldwide, the prevalence of migraines is explain the physiologic processes associated with the onset of increasing. Migraines are considered to be a severely migraine HAs. The most prominent are neuronal disabling condition. Migraines can onset due to noxious abnormalities, a platelet disorder, a deficiency of serotonin stimuli inducing flaccidity of the vasculature for 24-48 and mitochondrial dysfunction. All of these mechanisms can hours. There are a number of mechanisms that may be initiate an ischemic event. responsible. However, the most prominent are The neurological dysfunction experienced is due to a defect irregularities of the trigeminovascular system, a platelet in the trigeminovascular system, which causes an intense disorder, serotonin deficiency and mitochondrial depolarizing wave that suppresses the activity of the brain [4]. dysfunction. The addition of Curcuma domestica or Simultaneously substance P is released from the Zingiber officinale into the diet or supplementation with these herbs or magnesium may help to reduce the trigeminovascular system [5]. -

The Role of Cysteinyl Leukotrienes in Asthma: from the Molecule to the Bedside
Allergology International (1996) 45: 163-169 Review Article The role of cysteinyl leukotrienes in asthma: From the molecule to the bedside BEA Lams and TH Lee Department of Allergy and Respiratory Medicine, Guy's Hospital, London, UK ABSTRACT via LTC4synthase, to the cysteinyl leukotriene LTC4which is sub- sequently converted by y-glutamyltranspeptidase to LTD4and by There is increasing interest in the role played by the cysteinyl a dipeptidase to LTE4. leukotrienes in the pathogenesis of bronchial asthma. They have been demonstrated to have a bronchoconstrictor effect both in vitro and in vivo and have been isolated from bron- BRONCHOCONSTRICTOR EFFECT OF THE choalveolar lavage fluid and urine from stable asthmatics LEUKOTRIENES and from asthmatics during exacerbations and after endo- In vitro studies with LTC4have demonstrated a contractile action bronchial challenge. Pharmacological intervention has been on isolated human bronchus2 and tracheal smooth muscle3 with studied through antagonism of leukotriene receptors and a potency of 1000 times that of histamine. Isolated human inhibition of leukotriene synthesis. Both have been shown to bronchi of diameter 3-12mm have a high degree of baseline have an effect on the asthmatic response after challenge with bronchomotor tone equivalent to 50% of maximal broncho- allergen, exercise and inhalation of cold air and to have an constriction induced by. BaCI2. This resting tone is reduced by effect on aspirin sensitive asthmatics and on the symptoms preincubation with the LTD4antagonists ICI 204,219 and SKF and markers of severity of chronic asthma. The differences 104,353 and this reduction is additive to that induced by the between receptor antagonism and synthesis inhibition are addition of histamine Hl antagonists suggesting thatthis resting discussed.