Springer MRW: [AU:0, IDX:0]
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -
Gc Applications
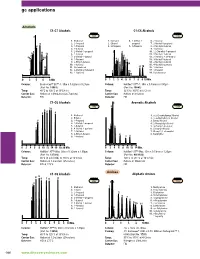
gc applications Alcohols C1-C7 Alcohols C1-C6 Alcohols 1007 1255 1. Methanol 1. Methanol 4. 2-Methyl-2- 6. 2-Butanol 2. 2-Propanol 2. Ethanol propanol 7. 2-Methyl-1-propanol 3. 1-Propanol 3. 2-Propanol 5. 1-Propanol 8. 2-Methyl-2-butanol 4. 2-Butanol 9. 1-Butanol 5. 2-Methyl-1-propanol 10. 2,2-Dimethyl-1-propanol 6. 1-Butanol 11. 3-Methyl-2-butanol 7. 3-Methyl-1-butanol 12. 2-Pentanol + 3-Pentanol 8. 1-Pentanol 13. 3-Methyl-1-butanol 9. 2-Ethyl-1-butanol 14. 2-Methyl-1-butanol 10. 1-Hexanol 15. 4-Methyl-2-pentanol 11. Cyclohexanol 16. 1-Pentanol 12. 3-Methylcyclohexanol 17. 1-Hexanol 13. 1-Heptanol 18. Cyclohexanol å-IN Column: Econo-Cap™ EC™-1, 30m x 0.32mm x 0.25µm Column: Heliflex® AT™-1, 30m x 0.53mm x 5.00µm (Part No. 19651) (Part No. 16843) Temp: 40°C to 120°C at 10°C/min Temp: 35°C to 100°C at 5°C/min Carrier Gas: Helium at 1.09mL/min (26.7cm/sec) Carrier Gas: Helium at 3mL/min Detector: FID Detector: FID C1-C6 Alcohols Aromatic Alcohols 2596 1249 1. Methanol 1. A,A-Dimethylbenzyl Alcohol 2. Ethanol 2. DL-A-Methylbenzyl Alcohol 3. 1-Propanol 3. Benzyl Alcohol 4. 2-Methyl-1-propanol 4. 2-Phenylethyl Alcohol 5. 1-Butanol 5. 3-Phenyl-1-propanol 6. 4-Methyl-2-pentanol 6. Cinnamyl Alcohol 7. 1-Pentanol 7. Phenyl-1,2-ethanediol 8. 2-Ethyl-1-butanol 8. -

Visible-Light-Driven Photooxidation of Alcohols Using Surface-Doped Graphitic Carbon Nitride
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is © The Royal Society of Chemistry 2017 Visible-Light-Driven Photooxidation of alcohols using surface-doped graphitic carbon nitride Wuyuan Zhang, Anna Bariotaki, Ioulia Smonou, and Frank Hollmann* Material and Methods Materials Unless stated otherwise all chemicals were purchased from Sigma-Aldrich, Fluka, Acros or Alfa-Aesar with the highest purity available and used without further treatment. Synthesis of g-C3N4 and carbon nanodots doped (CD-C3N4) The g-C3N4 was synthesized via the simple calcination (Carbolite furnace; CWF 12/5, 2400 W) of urea (10.0 g) at 600 °C for 4 h (5 °C/min), a yellowish powder was obtained after the cooling to room temperature.1 The carbon nanodots were synthesized via the thermal decomposition of sucrose with slight modification.2 Briefly, 0.75 g of sucrose was dissolved in 30 mL of MilliQ water and stirred at room temperature for 0.5 h. The solution was transferred into a 45 mL Teflon-lined stainless steel autoclave reactor, and heated at 180 °C for 5 h (10 °C/min). After cooling the reactor to room temperature, a brown mixture was obtained. The mixture was centrifuged (8000 rpm for 20 min), and the pellet was washed with water (3×) and freeze-dried. 3 In order to deposit carbon nanodots to the surface of g-C3N4, Liu et al’s procedures were adopted. Firstly, a stock solution of carbon nanodots (1 mg in 25mL water) was prepared. 15.0 mL of this stock solution was mixed with 15.0 mL of NH4OH (28 %) and sealed in a 45 mL Teflon-lined autoclave reactor. -

(12) United States Patent (10) Patent No.: US 7,576,170 B2 Perry Et Al
US00757617OB2 (12) United States Patent (10) Patent No.: US 7,576,170 B2 Perry et al. (45) Date of Patent: Aug. 18, 2009 (54) CYCLIC SILOXANE COMPOSITIONS FOR 6,046,156 A 4/2000 Perry THE RELEASE OF ACTIVE INGREDIENTS 6,054,547 A 4/2000 Perry et al. 6,063,365 A 5, 2000 Schefer et al. (75) Inventors: Robert J. Perry, Niskayuna, NY (US); 6,075,111 A 6/2000 Perry et al. Mark D. Leatherman, Elmsford, NY 6,077.923 A 6/2000 Perry et al. (US); Shahid Murtuza, Albany, NY 6,083,901 A 7/2000 Perry et al. (US) 6,121,343 A 9/2000 Hongo et al. 6,143,309 A 11/2000 Legrow et al. (73) Assignee: Momentive Performance Materials, 6,153,578 A 11/2000 Perry Albany, NY (US) 6,200,949 B1 3/2001 Reijmer et al. 6,228,380 B1 5, 2001 LeGrow et al. (*) Notice: Subject to any disclaimer, the term of this 6,262,287 B1 7/2001 Anderson et al. patent is extended or adjusted under 35 6,267,977 B1 7/2001 LeGrow et al. U.S.C. 154(b) by 993 days. 6,309,715 B1 10/2001 Lindauer et al. 6,322,777 B1 1 1/2001 Perry et al. (21) Appl. No.: 10/742,415 6,325,274 B2 12/2001 Esumi et al. 6,325,859 B1 12/2001 De Roos et al. (22) Filed: Dec. 19, 2003 6,435,423 B2 8/2002 Hurry et al. (65) Prior Publication Data 6,624,136 B2 9, 2003 Guerinet al. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group D07A (Corticosteroids, Plain) Table of Contents Page INTRODUCTION 4 DISCLAIMER 6 GLOSSARY OF TERMS USED IN THIS DOCUMENT 7 ACTIVE SUBSTANCES Methylprednisolone (ATC: D07AA01) 8 Hydrocortisone (ATC: D07AA02) 9 Prednisolone (ATC: D07AA03) 11 Clobetasone (ATC: D07AB01) 13 Hydrocortisone butyrate (ATC: D07AB02) 16 Flumetasone (ATC: D07AB03) 18 Fluocortin (ATC: D07AB04) 21 Fluperolone (ATC: D07AB05) 22 Fluorometholone (ATC: D07AB06) 23 Fluprednidene (ATC: D07AB07) 24 Desonide (ATC: D07AB08) 25 Triamcinolone (ATC: D07AB09) 27 Alclometasone (ATC: D07AB10) 29 Hydrocortisone buteprate (ATC: D07AB11) 31 Dexamethasone (ATC: D07AB19) 32 Clocortolone (ATC: D07AB21) 34 Combinations of Corticosteroids (ATC: D07AB30) 35 Betamethasone (ATC: D07AC01) 36 Fluclorolone (ATC: D07AC02) 39 Desoximetasone (ATC: D07AC03) 40 Fluocinolone Acetonide (ATC: D07AC04) 43 Fluocortolone (ATC: D07AC05) 46 2 Diflucortolone (ATC: D07AC06) 47 Fludroxycortide (ATC: D07AC07) 50 Fluocinonide (ATC: D07AC08) 51 Budesonide (ATC: D07AC09) 54 Diflorasone (ATC: D07AC10) 55 Amcinonide (ATC: D07AC11) 56 Halometasone (ATC: D07AC12) 57 Mometasone (ATC: D07AC13) 58 Methylprednisolone Aceponate (ATC: D07AC14) 62 Beclometasone (ATC: D07AC15) 65 Hydrocortisone Aceponate (ATC: D07AC16) 68 Fluticasone (ATC: D07AC17) 69 Prednicarbate (ATC: D07AC18) 73 Difluprednate (ATC: D07AC19) 76 Ulobetasol (ATC: D07AC21) 77 Clobetasol (ATC: D07AD01) 78 Halcinonide (ATC: D07AD02) 81 LIST OF AUTHORS 82 3 INTRODUCTION The availability of medicines with or without a medical prescription has implications on patient safety, accessibility of medicines to patients and responsible management of healthcare expenditure. The decision on prescription status and related supply conditions is a core competency of national health authorities. -

Subject Index
52_1107_1136_SI 16.11.2005 9:35 Uhr Seite 1107 Subject Index A – tape 264, 368, 940 α-adjustment 154 AAS, see atomic absorption spectrophotometry adrenocorticotrophic hormone (ACTH) 21 abietic acid 909, 943 adverse drug reaction 401 abrasion 174, 283 aeroallergen 391 absorption through appendage 169 – atopic eczema 391 α-acaridial 329 – avoidance 391 accident 889 aerospace 726 acebutolol hydrochloride 909 African aceclofenac 909 –ebony783 acetaldehyde 943 – mahagony 783 acetone 118, 666 – red padauk wood 783 acetylacetone 697 Agave acetylsalicylic acid 84, 909 – americana 354 Achillea millefolium (yarrow extract) 909 – tequilana 225 aciclovir 909 age 279 acid 110 agent orange 806 – black 48 (CI 65005) 909 AGEP 404 – dye 689 Agfa TSS 355 – halogenated 259 aggravation 204 –hydrochloric261 agricultural worker 272 –nitric261 agriculture 725 –red AICD, see activation-induced cell death – – 14 (azorubine) 909 airborne – – 118 (CI 26410) 909 – allergic contact dermatitis 218, 228, 315, 467, 477, 484, 598, ––359909 627, 654, 788 – violet 17 (CI 42650) 909 – contact urticaria 753, 758 – yellow – irritant contact dermatitis 625 – – 36 (CI 13065, metanil yellow) 909 aircraft manufacture 560 – – 61 (CI 18968) 909 airway symptom 520 acitretin 341 alachlor 953 acneiform alantolactone 55, 789, 909, 954 – folliculitis 229 alclometasone-17,21-dipropionate 909 –lesion265 alclometasone-17-propionate 58 acrodermatitis enteropathica 241 alcohol, see also ethyl 909 acrovesicular dermatitis 401 aldehyde 110, 607, 886 acrylamide 592, 944 algicide 562 acrylate -

Synthesis and Characterization of Zinc Ii Complexes with S Alkyl Derivatives of Thiosalicylic Acid Sinteza I Karakteriz
ORIGINAL SCIENTIFIC PAPER ORIGINALNI NAUČNI RAD ORIGINAL SCIENTIFIC PAPER SYNTHESIS AND CHARACTERIZATION OF ZINCIICOMPLEXES WITH SALKYL DERIVATIVES OF THIOSALICYLIC ACID Milos V. Nikolic1, Marina Z. Mijajlovic1, Dusan Lj. Tomovic1, Andriana M. Bukonjic1, Verica V. Jevtic2, Zoran R. Ratkovic2, Srecko R. Trifunovic2, Gordana P. Radic1 1University of Kragujevac, Serbia, Faculty of Medical Sciences, Department of Pharmacy 2University of Kragujevac, Serbia, Faculty of Science, Department of Chemistry SINTEZA I KARAKTERIZACIJA CINKIIKOMPLEKSA SA NEKIM SALKIL DERIVATIMA TIOSALICILNE KISELINE Miloš V. Nikolić1, Marina Ž. Mijajlović1, Dušan Lj. Tomović1, Andriana M. Bukonjić1, Verica V. Jevtić2, Zoran R. Ratković2, Srećko R. Trifunović2, Gordana P. Radić1 1Univerzitet u Kragujevcu, Srbija, Fakultet medicinskih nauka, Odsek za farmaciju 2 Univerzitet u Kragujevcu, Srbija, Prirodno-matematički fakultet, Institut za hemiju Received / Primljen: 02.02.2017. Accepted / Prihvaćen: 12.02.2017. ABSTRACT SAŽETAK New zinc(II)-complexes with S-alkyl derivatives of thio- Novi cink(II)-kompleksi sa S-alkil derivatima tiosalicil- salicylic acid (alkyl = benzyl-(L1), methyl-(L2), ethyl-(L3), ne kiseline (alkil = benzil-(L1), metil-(L2), etil-(L3), pro- propyl-(L4), butyl-(L5)) have been synthesized and charac- pil-(L4), butil-(L5)) su sintetisani i okarakterisani na osno- terized by elemental microanalysis, IR spectroscopy, and 1H vu rezultata elementalne mikroanalize, IR, 1H i 13C NMR and 13C NMR spectroscopy. Th e S-alkyl derivatives of thio- spektroskopije. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

A Practical and Stereoselective Synthesis of Cinnamyl Alcohols Bearing A-Cyano Or A-Ester Functional Group from Baylis-Hillman Adducts
Notes Bull. Korean Chem. Soc. 2004, Vol. 25, No. 3 413 A Practical and Stereoselective Synthesis of Cinnamyl Alcohols Bearing a-Cyano or a-Ester Functional Group from Baylis-Hillman Adducts Ka Young Lee, Saravanan GowriSankar, and Jae Nyoung Kim Department of Chemistry and Institute of Basic Science, Chonnam National University, Gwangju 500-757, Korea Received December 18, 2003 Key Words : Cinnamyl alcohols, Baylis-Hillman adducts, Sulfuric acid, Acetic anhydride Cinnamyl alcohols bearing ester, acid, or nitrile functional Hillman alcohol into the primary acetate or eventually to group at the 2-position are very useful synthons for the primary alcohol (Scheme 1 and Table 1). synthesis of various biologically important compounds.1 The reaction of Baylis-Hillman adducts with acetic Synthesis of cinnamyl alcohol derivatives from the Baylis- anhydride (1.5 equiv.) in the presence of sulfuric acid (10 Hillman adducts has been reported by us and other groups.1-4 mol%) gave the corresponding rearranged cinnamyl acetates The conversion can be carried out directly by using 20% quantitatively in dichloromethane at room temperature aqueous sulfuric acid (for the Baylis-Hillman adducts within 30 min (Actually, the cinnamyl acetate derivatives derived from acrylonitrile)2 or trifluoroacetic acid (for the could be isolated in 92-95% yields.). In order to obtain the Baylis-Hillman adducts derived from acrylates).3 Recently, corresponding cinnamyl alcohols in a one-pot we tried some Basavaiah and coworkers have reported the two-step, one- reaction conditions for the next partial hydrolysis. Addition pot conversion method: TMSOTf-assisted conversion of of water to the reaction mixture (two-phase system) afforded Baylis-Hillman alcohol into the corresponding primary the hydrolyzed cinnamyl alcohols in trace amounts. -

Premenstrual Syndrome: a Natural Approach to Management
CNI506 8/99 Vol. 5, No. 6 APPLIED NUTRITIONAL SCIENCE REPORTS Copyright © 1997 Advanced Nutrition Publications, Inc. rev. 1999 Premenstrual Syndrome: A Natural Approach to Management BY JOSEPH L. MAYO, MD, FACOG ABSTRACT: Premenstrual syndrome (PMS) is a disorder that imbalances, nutritional insufficiencies, and psychologic factors. occurs during the luteal phase of the menstrual cycle, producing A nutritional approach to PMS that takes into account the complex a diverse number of physical and emotional changes. The most interactions of all bodily systems that influence hormonal balance common symptoms of PMS include bloating, backache, breast and neuroendocrine function, with an emphasis on the liver, is tenderness, food cravings, fatigue, irritability, and depression. recommended. The nutritional factors that have been studied The timing of the appearance and disappearance of symptoms, include vitamin B6, magnesium, zinc, choline, vitamin E, and rather than the presence of specific symptoms, is of more essential fatty acids, in addition to weight management and importance in the diagnosis of PMS. The direct cause of PMS is stress reduction. Herbal therapies have also proven beneficial in unknown, although there are numerous theories relating to hormonal the management of PMS. PREMENSTRUAL SYNDROME symptoms such as bloating, breast tenderness, and headache (Table 1).3-5 These diverse symptoms may range from mild Cyclic symptoms in women of reproductive age have been to incapacitating. In some women a single symptom, such recognized for thousands of years. First appearing in the medical as depression, may predominate, whereas others may have literature in 1931 and originally termed “premenstrual tension,” several symptoms.1 this condition has been renamed “premenstrual syndrome” (PMS) in an effort to take into account the different clinical Table. -

In Chemistry, Glycosides Are Certain Molecules in Which a Sugar Part Is
GLYCOSIDES Glycosides may be defined as the organic compounds from plants or animal sources, which on enzymatic or acid hydrolysis give one or more sugar moieties along with non- sugar moiety. Glycosides play numerous important roles in living organisms. Many plants store important chemicals in the form of inactive glycosides; if these chemicals are needed, the glycosides are brought in contact with water and an enzyme, and the sugar part is broken off, making the chemical available for use. Many such plant glycosides are used as medications. In animals (including humans), poisons are often bound to sugar molecules in order to remove them from the body. Formally, a glycoside is any molecule in which a sugar group is bonded through its carbon atom to another group via an O-glycosidic bond or an S-glycosidic bond; glycosides involving the latter are also called thioglycosides. The sugar group is then known as the glycone and the non-sugar group as the aglycone or genin part of the glycoside. The glycone can consist of a single sugar group (monosaccharide) or several sugar groups (oligosaccharide). Classification Classification based on linkages Based on the linkage of sugar moiety to aglycone part 1. O-Glycoside:-Here the sugar is combined with alcoholic or phenolic hydroxyl function of aglycone.eg:-digitalis. 2. N-glycosides:-Here nitrogen of amino group is condensed with a sugar ,eg- Nucleoside 3. S-glycoside:-Here sugar is combined with sulphur of aglycone,eg- isothiocyanate glycosides. 4. C-glycosides:-By condensation of a sugar with a cabon atom, eg-Cascaroside, aloin. Glycosides can be classified by the glycone, by the type of glycosidic bond, and by the aglycone. -

The Phytochemistry of Cherokee Aromatic Medicinal Plants
medicines Review The Phytochemistry of Cherokee Aromatic Medicinal Plants William N. Setzer 1,2 1 Department of Chemistry, University of Alabama in Huntsville, Huntsville, AL 35899, USA; [email protected]; Tel.: +1-256-824-6519 2 Aromatic Plant Research Center, 230 N 1200 E, Suite 102, Lehi, UT 84043, USA Received: 25 October 2018; Accepted: 8 November 2018; Published: 12 November 2018 Abstract: Background: Native Americans have had a rich ethnobotanical heritage for treating diseases, ailments, and injuries. Cherokee traditional medicine has provided numerous aromatic and medicinal plants that not only were used by the Cherokee people, but were also adopted for use by European settlers in North America. Methods: The aim of this review was to examine the Cherokee ethnobotanical literature and the published phytochemical investigations on Cherokee medicinal plants and to correlate phytochemical constituents with traditional uses and biological activities. Results: Several Cherokee medicinal plants are still in use today as herbal medicines, including, for example, yarrow (Achillea millefolium), black cohosh (Cimicifuga racemosa), American ginseng (Panax quinquefolius), and blue skullcap (Scutellaria lateriflora). This review presents a summary of the traditional uses, phytochemical constituents, and biological activities of Cherokee aromatic and medicinal plants. Conclusions: The list is not complete, however, as there is still much work needed in phytochemical investigation and pharmacological evaluation of many traditional herbal medicines. Keywords: Cherokee; Native American; traditional herbal medicine; chemical constituents; pharmacology 1. Introduction Natural products have been an important source of medicinal agents throughout history and modern medicine continues to rely on traditional knowledge for treatment of human maladies [1]. Traditional medicines such as Traditional Chinese Medicine [2], Ayurvedic [3], and medicinal plants from Latin America [4] have proven to be rich resources of biologically active compounds and potential new drugs.