Research/Investigación First Comprehensive Molecular
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Shell Morphology, Radula and Genital Structures of New Invasive Giant African Land
bioRxiv preprint doi: https://doi.org/10.1101/2019.12.16.877977; this version posted December 16, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Shell Morphology, Radula and Genital Structures of New Invasive Giant African Land 2 Snail Species, Achatina fulica Bowdich, 1822,Achatina albopicta E.A. Smith (1878) and 3 Achatina reticulata Pfeiffer 1845 (Gastropoda:Achatinidae) in Southwest Nigeria 4 5 6 7 8 9 Alexander B. Odaibo1 and Suraj O. Olayinka2 10 11 1,2Department of Zoology, University of Ibadan, Ibadan, Nigeria 12 13 Corresponding author: Alexander B. Odaibo 14 E.mail :[email protected] (AB) 15 16 17 18 1 bioRxiv preprint doi: https://doi.org/10.1101/2019.12.16.877977; this version posted December 16, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 19 Abstract 20 The aim of this study was to determine the differences in the shell, radula and genital 21 structures of 3 new invasive species, Achatina fulica Bowdich, 1822,Achatina albopicta E.A. 22 Smith (1878) and Achatina reticulata Pfeiffer, 1845 collected from southwestern Nigeria and to 23 determine features that would be of importance in the identification of these invasive species in 24 Nigeria. -

The Malacological Society of London
ACKNOWLEDGMENTS This meeting was made possible due to generous contributions from the following individuals and organizations: Unitas Malacologica The program committee: The American Malacological Society Lynn Bonomo, Samantha Donohoo, The Western Society of Malacologists Kelly Larkin, Emily Otstott, Lisa Paggeot David and Dixie Lindberg California Academy of Sciences Andrew Jepsen, Nick Colin The Company of Biologists. Robert Sussman, Allan Tina The American Genetics Association. Meg Burke, Katherine Piatek The Malacological Society of London The organizing committee: Pat Krug, David Lindberg, Julia Sigwart and Ellen Strong THE MALACOLOGICAL SOCIETY OF LONDON 1 SCHEDULE SUNDAY 11 AUGUST, 2019 (Asilomar Conference Center, Pacific Grove, CA) 2:00-6:00 pm Registration - Merrill Hall 10:30 am-12:00 pm Unitas Malacologica Council Meeting - Merrill Hall 1:30-3:30 pm Western Society of Malacologists Council Meeting Merrill Hall 3:30-5:30 American Malacological Society Council Meeting Merrill Hall MONDAY 12 AUGUST, 2019 (Asilomar Conference Center, Pacific Grove, CA) 7:30-8:30 am Breakfast - Crocker Dining Hall 8:30-11:30 Registration - Merrill Hall 8:30 am Welcome and Opening Session –Terry Gosliner - Merrill Hall Plenary Session: The Future of Molluscan Research - Merrill Hall 9:00 am - Genomics and the Future of Tropical Marine Ecosystems - Mónica Medina, Pennsylvania State University 9:45 am - Our New Understanding of Dead-shell Assemblages: A Powerful Tool for Deciphering Human Impacts - Sue Kidwell, University of Chicago 2 10:30-10:45 -

Achatina Fulica and Archachatina Marginata Was Sampled in the Littoral, Center and West Regions of Cameroon
Central Journal of Veterinary Medicine and Research Research Article *Corresponding author Prisca Meffowoet, Faculty of Agronomy and Agricul- tural Sciences, University of Dschang, Dschang, Tel: Infestation rate of African 699366311/676879291; Email: [email protected] Submitted: 25 March 2020 giant snails (Achatina fulica Accepted: 02 April 2020 Published: 06 April 2020 ISSN: 2379-948X and Archachatina marginata) by Copyright © 2020 Prisca MC, et al. parasites during the rainy season OPEN ACCESS Keywords • Cameroon in three localities of Cameroon • African giant snails • Parasites Meffowoet CP1*, Kouam KM1, Kana JR2, and Tchakounte FM2 1Animal Physiology and Health Research Unit, University of Dschang, Cameroon 2Animal Nutrition and Production Research Unit, University of Dschang, Cameroon Abstract This study was designed during the rainy season in order to identify the parasites likely to infest edible snails. 360 Achatina fulica and Archachatina marginata was sampled in the Littoral, Center and West regions of Cameroon. After macroscopic observation of snails, the hepatopancreas, digestive tract, sex organs, slime and haemolymph were isolated. These samples were examined using the flotation techniques and direct rubbing. Of the 360 snails sampled, 213 (59.3%) were infested, that is 147 (82.1%) for A. marginata and 66 (36.7%) for A. fulica respectively. The highest infestation rate was recorded on protozoans (41.4%) followed by nematode (24.7%). The most represented parasites were Trichodina achatinae (23.9%) and Strongyloides stercoralis (16.1%); while the least represented were cyst of Balantidium coli (8.1%), Enteromonas sp. (8.1%), cyst of Isospora sp. (7.8%), larva of Protostrongylus sp. (7.5%), cyst of Cryptosporidium sp. -

An Assessment of Land and Aquatic Snails in the South African Pet Trade
Management of Biological Invasions (2020) Volume 11, Issue 3: 512–524 CORRECTED PROOF Research Article Exotic gastropods for sale: an assessment of land and aquatic snails in the South African pet trade Tinyiko C. Shivambu, Ndivhuwo Shivambu and Colleen T. Downs* Centre for Excellence in Invasion Biology, and Centre for Functional Biodiversity, School of Life Sciences, University of KwaZulu-Natal, Private Bag X01, Scottsville, Pietermaritzburg, 3209, South Africa Author e-mails: [email protected] (CTD), [email protected] (TCS), [email protected] (NS) *Corresponding author Citation: Shivambu TC, Shivambu N, Downs CT (2020) Exotic gastropods for Abstract sale: an assessment of land and aquatic snails in the South African pet trade. Gastropods are amongst the most popular of the Mollusca in the pet trade, with Management of Biological Invasions 11(3): globalisation being the main contributing factor facilitating their establishment 512–524, https://doi.org/10.3391/mbi.2020.11.3.11 globally. Although it is known that gastropods are kept as pets in South Africa, Received: 27 February 2020 relatively little has been documented on the trade for this group. Physical pet stores Accepted: 20 May 2020 selling gastropod species were surveyed seasonally in South Africa, aiming to determine 1) the types of species sold, including their trade popularity, trade volume, Published: 1 August 2020 and the biogeographic realms they originated from, and 2) seasonal variations in Handling editor: Ana Novoa gastropod species traded. Six gastropod species were recorded in the South African Thematic editor: Catherine Jarnevich pet stores with three known invasives (Achatina fulica, A. immaculata, and Pomacea Copyright: © Shivambu et al. -

The Gastropod Shell Has Been Co-Opted to Kill Parasitic Nematodes
www.nature.com/scientificreports OPEN The gastropod shell has been co- opted to kill parasitic nematodes R. Rae Exoskeletons have evolved 18 times independently over 550 MYA and are essential for the success of Received: 23 March 2017 the Gastropoda. The gastropod shell shows a vast array of different sizes, shapes and structures, and Accepted: 18 May 2017 is made of conchiolin and calcium carbonate, which provides protection from predators and extreme Published: xx xx xxxx environmental conditions. Here, I report that the gastropod shell has another function and has been co-opted as a defense system to encase and kill parasitic nematodes. Upon infection, cells on the inner layer of the shell adhere to the nematode cuticle, swarm over its body and fuse it to the inside of the shell. Shells of wild Cepaea nemoralis, C. hortensis and Cornu aspersum from around the U.K. are heavily infected with several nematode species including Caenorhabditis elegans. By examining conchology collections I show that nematodes are permanently fixed in shells for hundreds of years and that nematode encapsulation is a pleisomorphic trait, prevalent in both the achatinoid and non-achatinoid clades of the Stylommatophora (and slugs and shelled slugs), which diverged 90–130 MYA. Taken together, these results show that the shell also evolved to kill parasitic nematodes and this is the only example of an exoskeleton that has been co-opted as an immune system. The evolution of the shell has aided in the success of the Gastropoda, which are composed of 65–80,000 spe- cies that have colonised terrestrial and marine environments over 400MY1, 2. -

Abstract Volume
ABSTRACT VOLUME August 11-16, 2019 1 2 Table of Contents Pages Acknowledgements……………………………………………………………………………………………...1 Abstracts Symposia and Contributed talks……………………….……………………………………………3-225 Poster Presentations…………………………………………………………………………………226-291 3 Venom Evolution of West African Cone Snails (Gastropoda: Conidae) Samuel Abalde*1, Manuel J. Tenorio2, Carlos M. L. Afonso3, and Rafael Zardoya1 1Museo Nacional de Ciencias Naturales (MNCN-CSIC), Departamento de Biodiversidad y Biologia Evolutiva 2Universidad de Cadiz, Departamento CMIM y Química Inorgánica – Instituto de Biomoléculas (INBIO) 3Universidade do Algarve, Centre of Marine Sciences (CCMAR) Cone snails form one of the most diverse families of marine animals, including more than 900 species classified into almost ninety different (sub)genera. Conids are well known for being active predators on worms, fishes, and even other snails. Cones are venomous gastropods, meaning that they use a sophisticated cocktail of hundreds of toxins, named conotoxins, to subdue their prey. Although this venom has been studied for decades, most of the effort has been focused on Indo-Pacific species. Thus far, Atlantic species have received little attention despite recent radiations have led to a hotspot of diversity in West Africa, with high levels of endemic species. In fact, the Atlantic Chelyconus ermineus is thought to represent an adaptation to piscivory independent from the Indo-Pacific species and is, therefore, key to understanding the basis of this diet specialization. We studied the transcriptomes of the venom gland of three individuals of C. ermineus. The venom repertoire of this species included more than 300 conotoxin precursors, which could be ascribed to 33 known and 22 new (unassigned) protein superfamilies, respectively. Most abundant superfamilies were T, W, O1, M, O2, and Z, accounting for 57% of all detected diversity. -

Pest Alert: Giant African Snails
Pest Alert Giant African Snails “Giant African snail” is the Services. This action resulted common name used to describe in the State’s second giant several foreign snail species African snail detection. The that could become serious U.S. Department of Agriculture agricultural pests in the United (USDA) and Florida have States. The most important giant invested several million dollars African snail is Lissachatina since this second detection, fulica (formerly Achatina fulica). and work continues today to Figure 1. A mature Lissachatina fulica eradicate this pest. maneuvers in its environment. The Giant African Snail Description/Life Cycle Scientists consider L. fulica to be one of the most damaging Reaching almost 8 inches land snails in the world. It is (20 centimeters) in length and known to feed on at least 5 inches (13 centimeters) in 500 different types of plants, diameter, L. fulica is one of the including peanuts, beans, peas, world’s largest land snails— cucumbers, and melons. If about the size of an average fruits and vegetables are not adult fist. When fully grown, available, they will eat a wide its shell consists of seven to variety of ornamental plants, tree nine whorls, with a long and bark, and even paint and stucco greatly swollen body whorl. The Figure 2. A penny is used to show the size of on houses. giant African snail eggs. brownish shell with darker brown lengthwise stripes covers at L. fulica is established least half the length of the snail. throughout the Indo-Pacific Florida and the Giant basin, from east Africa to African Snail Each snail contains both Hawaii and Guam, including female and male reproductive the Southern Asian region. -

Biosecurity Risk Assessment
An Invasive Risk Assessment Framework for New Animal and Plant-based Production Industries RIRDC Publication No. 11/141 RIRDCInnovation for rural Australia An Invasive Risk Assessment Framework for New Animal and Plant-based Production Industries by Dr Robert C Keogh February 2012 RIRDC Publication No. 11/141 RIRDC Project No. PRJ-007347 © 2012 Rural Industries Research and Development Corporation. All rights reserved. ISBN 978-1-74254-320-8 ISSN 1440-6845 An Invasive Risk Assessment Framework for New Animal and Plant-based Production Industries Publication No. 11/141 Project No. PRJ-007347 The information contained in this publication is intended for general use to assist public knowledge and discussion and to help improve the development of sustainable regions. You must not rely on any information contained in this publication without taking specialist advice relevant to your particular circumstances. While reasonable care has been taken in preparing this publication to ensure that information is true and correct, the Commonwealth of Australia gives no assurance as to the accuracy of any information in this publication. The Commonwealth of Australia, the Rural Industries Research and Development Corporation (RIRDC), the authors or contributors expressly disclaim, to the maximum extent permitted by law, all responsibility and liability to any person, arising directly or indirectly from any act or omission, or for any consequences of any such act or omission, made in reliance on the contents of this publication, whether or not caused by any negligence on the part of the Commonwealth of Australia, RIRDC, the authors or contributors. The Commonwealth of Australia does not necessarily endorse the views in this publication. -

Snail Mucus from the Mantle and Foot of Two Land
Noothuan et al. BMC Res Notes (2021) 14:138 https://doi.org/10.1186/s13104-021-05557-0 BMC Research Notes RESEARCH NOTE Open Access Snail mucus from the mantle and foot of two land snails, Lissachatina fulica and Hemiplecta distincta, exhibits diferent protein profle and biological activity Nattaphop Noothuan1, Kantamas Apitanyasai1, Somsak Panha2 and Anchalee Tassanakajon1* Abstract Objective: Snails secrete diferent types of mucus that serve several functions, and are increasingly being exploited for medical and cosmetic applications. In this study, we explored the protein pattern and compared the biological properties of the mucus secreted from the mantle collar and foot of two snail species, Lissachatina fulica and Hemi- plecta distincta. Result: Protein profle showed a diferent pattern between the two species and between the two secretory parts. The mantle-specifc protein bands were further characterized and among them was an antibacterial protein, achacin. Accordingly, the mucus from the mantle exhibited the higher antibacterial activity than that from the foot in both snail species. The mucus from H. distincta, frst reported here, also showed antibacterial properties, but with a lower activity compared to that for L. fulica. Snail mucus also exhibited anti-tyrosinase activity and antioxidant activity but with no signifcant diference between the foot and mantle mucus. These results indicate some diferent protein compositions and biological activities of snail slime from the mantle and foot, which might be associated with their specifc functions in the animal and are useful for medical applications. Keywords: Land snail, Snail mucus, Antimicrobial activity, Anti-tyrosinase activity, Antioxidant activity Introduction compounds have been found in snail mucus, such as Land snails have been used as food and for various allantoin, hyaluronic acid, peptides and proteins [3, 4, medical treatments for centuries [1–3]. -

Giant African Snail Cooperative Eradication Program
United States Department of Agriculture Giant African Snail Marketing and Regulatory Cooperative Programs Animal and Eradication Program Plant Health Inspection Service Miami-Dade County, Florida Environmental Assessment, October 2011 Giant African Snail Cooperative Eradication Program Environmental Assessment October 2011 Agency Contact: Andrea Simao USDA–APHIS–PPQ Emergency and Domestic Programs 4700 River Road, Unit 26 Riverdale, MD 20737 __________________________________________________________ The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’S TARGET Center at (202) 720–2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326–W, Whitten Building, 1400 Independence Avenue, SW, Washington, DC 20250–9410 or call (202) 720–5964 (voice and TDD). USDA is an equal opportunity provider and employer. __________________________________________________________ Mention of companies or commercial products in this report does not imply recommendation or endorsement by the U.S. Department of Agriculture over others not mentioned. USDA neither guarantees nor warrants the standard of any product -
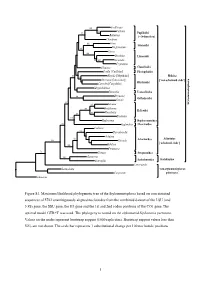
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia -

Life After Logging: Reconciling Wildlife Conservation and Production Forestry in Indonesian Borneo
Life after logging Reconciling wildlife conservation and production forestry in Indonesian Borneo Erik Meijaard • Douglas Sheil • Robert Nasi • David Augeri • Barry Rosenbaum Djoko Iskandar • Titiek Setyawati • Martjan Lammertink • Ike Rachmatika • Anna Wong Tonny Soehartono • Scott Stanley • Timothy O’Brien Foreword by Professor Jeffrey A. Sayer Life after logging: Reconciling wildlife conservation and production forestry in Indonesian Borneo Life after logging: Reconciling wildlife conservation and production forestry in Indonesian Borneo Erik Meijaard Douglas Sheil Robert Nasi David Augeri Barry Rosenbaum Djoko Iskandar Titiek Setyawati Martjan Lammertink Ike Rachmatika Anna Wong Tonny Soehartono Scott Stanley Timothy O’Brien With further contributions from Robert Inger, Muchamad Indrawan, Kuswata Kartawinata, Bas van Balen, Gabriella Fredriksson, Rona Dennis, Stephan Wulffraat, Will Duckworth and Tigga Kingston © 2005 by CIFOR and UNESCO All rights reserved. Published in 2005 Printed in Indonesia Printer, Jakarta Design and layout by Catur Wahyu and Gideon Suharyanto Cover photos (from left to right): Large mature trees found in primary forest provide various key habitat functions important for wildlife. (Photo by Herwasono Soedjito) An orphaned Bornean Gibbon (Hylobates muelleri), one of the victims of poor-logging and illegal hunting. (Photo by Kimabajo) Roads lead to various impacts such as the fragmentation of forest cover and the siltation of stream— other impacts are associated with improved accessibility for people. (Photo by Douglas Sheil) This book has been published with fi nancial support from UNESCO, ITTO, and SwedBio. The authors are responsible for the choice and presentation of the facts contained in this book and for the opinions expressed therein, which are not necessarily those of CIFOR, UNESCO, ITTO, and SwedBio and do not commit these organisations.