Microbial Eukaryote Diversity in the Marine Oxygen Minimum Zone Off Northern Chile
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
Molecular Data and the Evolutionary History of Dinoflagellates by Juan Fernando Saldarriaga Echavarria Diplom, Ruprecht-Karls-Un
Molecular data and the evolutionary history of dinoflagellates by Juan Fernando Saldarriaga Echavarria Diplom, Ruprecht-Karls-Universitat Heidelberg, 1993 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES Department of Botany We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA November 2003 © Juan Fernando Saldarriaga Echavarria, 2003 ABSTRACT New sequences of ribosomal and protein genes were combined with available morphological and paleontological data to produce a phylogenetic framework for dinoflagellates. The evolutionary history of some of the major morphological features of the group was then investigated in the light of that framework. Phylogenetic trees of dinoflagellates based on the small subunit ribosomal RNA gene (SSU) are generally poorly resolved but include many well- supported clades, and while combined analyses of SSU and LSU (large subunit ribosomal RNA) improve the support for several nodes, they are still generally unsatisfactory. Protein-gene based trees lack the degree of species representation necessary for meaningful in-group phylogenetic analyses, but do provide important insights to the phylogenetic position of dinoflagellates as a whole and on the identity of their close relatives. Molecular data agree with paleontology in suggesting an early evolutionary radiation of the group, but whereas paleontological data include only taxa with fossilizable cysts, the new data examined here establish that this radiation event included all dinokaryotic lineages, including athecate forms. Plastids were lost and replaced many times in dinoflagellates, a situation entirely unique for this group. Histones could well have been lost earlier in the lineage than previously assumed. -

Molecular Phylogenetic Position of Hexacontium Pachydermum Jørgensen (Radiolaria)
Marine Micropaleontology 73 (2009) 129–134 Contents lists available at ScienceDirect Marine Micropaleontology journal homepage: www.elsevier.com/locate/marmicro Molecular phylogenetic position of Hexacontium pachydermum Jørgensen (Radiolaria) Tomoko Yuasa a,⁎, Jane K. Dolven b, Kjell R. Bjørklund b, Shigeki Mayama c, Osamu Takahashi a a Department of Astronomy and Earth Sciences, Tokyo Gakugei University, Koganei, Tokyo 184-8501, Japan b Natural History Museum, University of Oslo, P.O. Box 1172, Blindern, 0318 Oslo, Norway c Department of Biology, Tokyo Gakugei University, Koganei, Tokyo 184-8501, Japan article info abstract Article history: The taxonomic affiliation of Hexacontium pachydermum Jørgensen, specifically whether it belongs to the Received 9 April 2009 order Spumellarida or the order Entactinarida, is a subject of ongoing debate. In this study, we sequenced the Received in revised form 3 August 2009 18S rRNA gene of H. pachydermum and of three spherical spumellarians of Cladococcus viminalis Haeckel, Accepted 7 August 2009 Arachnosphaera myriacantha Haeckel, and Astrosphaera hexagonalis Haeckel. Our molecular phylogenetic analysis revealed that the spumellarian species of C. viminalis, A. myriacantha, and A. hexagonalis form a Keywords: monophyletic group. Moreover, this clade occupies a sister position to the clade comprising the spongodiscid Radiolaria fi Entactinarida spumellarians, coccodiscid spumellarians, and H. pachydermum. This nding is contrary to the results of Spumellarida morphological studies based on internal spicular morphology, placing H. pachydermum in the order Nassellarida Entactinarida, which had been considered to have a common ancestor shared with the nassellarians. 18S rRNA gene © 2009 Elsevier B.V. All rights reserved. Molecular phylogeny. 1. Introduction the order Entactinarida has an inner spicular system homologenous with that of the order Nassellarida. -

Broadly Sampled Multigene Analyses Yield a Well-Resolved Eukaryotic Tree of Life
Smith ScholarWorks Biological Sciences: Faculty Publications Biological Sciences 10-1-2010 Broadly Sampled Multigene Analyses Yield a Well-Resolved Eukaryotic Tree of Life Laura Wegener Parfrey University of Massachusetts Amherst Jessica Grant Smith College Yonas I. Tekle Smith College Erica Lasek-Nesselquist Marine Biological Laboratory Hilary G. Morrison Marine Biological Laboratory See next page for additional authors Follow this and additional works at: https://scholarworks.smith.edu/bio_facpubs Part of the Biology Commons Recommended Citation Parfrey, Laura Wegener; Grant, Jessica; Tekle, Yonas I.; Lasek-Nesselquist, Erica; Morrison, Hilary G.; Sogin, Mitchell L.; Patterson, David J.; and Katz, Laura A., "Broadly Sampled Multigene Analyses Yield a Well-Resolved Eukaryotic Tree of Life" (2010). Biological Sciences: Faculty Publications, Smith College, Northampton, MA. https://scholarworks.smith.edu/bio_facpubs/126 This Article has been accepted for inclusion in Biological Sciences: Faculty Publications by an authorized administrator of Smith ScholarWorks. For more information, please contact [email protected] Authors Laura Wegener Parfrey, Jessica Grant, Yonas I. Tekle, Erica Lasek-Nesselquist, Hilary G. Morrison, Mitchell L. Sogin, David J. Patterson, and Laura A. Katz This article is available at Smith ScholarWorks: https://scholarworks.smith.edu/bio_facpubs/126 Syst. Biol. 59(5):518–533, 2010 c The Author(s) 2010. Published by Oxford University Press, on behalf of the Society of Systematic Biologists. All rights reserved. For Permissions, please email: [email protected] DOI:10.1093/sysbio/syq037 Advance Access publication on July 23, 2010 Broadly Sampled Multigene Analyses Yield a Well-Resolved Eukaryotic Tree of Life LAURA WEGENER PARFREY1,JESSICA GRANT2,YONAS I. TEKLE2,6,ERICA LASEK-NESSELQUIST3,4, 3 3 5 1,2, HILARY G. -

The Planktonic Protist Interactome: Where Do We Stand After a Century of Research?
bioRxiv preprint doi: https://doi.org/10.1101/587352; this version posted May 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Bjorbækmo et al., 23.03.2019 – preprint copy - BioRxiv The planktonic protist interactome: where do we stand after a century of research? Marit F. Markussen Bjorbækmo1*, Andreas Evenstad1* and Line Lieblein Røsæg1*, Anders K. Krabberød1**, and Ramiro Logares2,1** 1 University of Oslo, Department of Biosciences, Section for Genetics and Evolutionary Biology (Evogene), Blindernv. 31, N- 0316 Oslo, Norway 2 Institut de Ciències del Mar (CSIC), Passeig Marítim de la Barceloneta, 37-49, ES-08003, Barcelona, Catalonia, Spain * The three authors contributed equally ** Corresponding authors: Ramiro Logares: Institute of Marine Sciences (ICM-CSIC), Passeig Marítim de la Barceloneta 37-49, 08003, Barcelona, Catalonia, Spain. Phone: 34-93-2309500; Fax: 34-93-2309555. [email protected] Anders K. Krabberød: University of Oslo, Department of Biosciences, Section for Genetics and Evolutionary Biology (Evogene), Blindernv. 31, N-0316 Oslo, Norway. Phone +47 22845986, Fax: +47 22854726. [email protected] Abstract Microbial interactions are crucial for Earth ecosystem function, yet our knowledge about them is limited and has so far mainly existed as scattered records. Here, we have surveyed the literature involving planktonic protist interactions and gathered the information in a manually curated Protist Interaction DAtabase (PIDA). In total, we have registered ~2,500 ecological interactions from ~500 publications, spanning the last 150 years. -

Metagenomic Characterization of Unicellular Eukaryotes in the Urban Thessaloniki Bay
Metagenomic characterization of unicellular eukaryotes in the urban Thessaloniki Bay George Tsipas SCHOOL OF ECONOMICS, BUSINESS ADMINISTRATION & LEGAL STUDIES A thesis submitted for the degree of Master of Science (MSc) in Bioeconomy Law, Regulation and Management May, 2019 Thessaloniki – Greece George Tsipas ’’Metagenomic characterization of unicellular eukaryotes in the urban Thessaloniki Bay’’ Student Name: George Tsipas SID: 268186037282 Supervisor: Prof. Dr. Savvas Genitsaris I hereby declare that the work submitted is mine and that where I have made use of another’s work, I have attributed the source(s) according to the Regulations set in the Student’s Handbook. May, 2019 Thessaloniki - Greece Page 2 of 63 George Tsipas ’’Metagenomic characterization of unicellular eukaryotes in the urban Thessaloniki Bay’’ 1. Abstract The present research investigates through metagenomics sequencing the unicellular protistan communities in Thermaikos Gulf. This research analyzes the diversity, composition and abundance in this marine environment. Water samples were collected monthly from April 2017 to February 2018 in the port of Thessaloniki (Harbor site, 40o 37’ 55 N, 22o 56’ 09 E). The extraction of DNA was completed as well as the sequencing was performed, before the downstream read processing and the taxonomic classification that was assigned using PR2 database. A total of 1248 Operational Taxonomic Units (OTUs) were detected but only 700 unicellular eukaryotes were analyzed, excluding unclassified OTUs, Metazoa and Streptophyta. In this research-based study the most abundant and diverse taxonomic groups were Dinoflagellata and Protalveolata. Specifically, the most abundant groups of all samples are Dinoflagellata with 190 OTUs (27.70%), Protalveolata with 139 OTUs (20.26%) Ochrophyta with 73 OTUs (10.64%), Cercozoa with 67 OTUs (9.77%) and Ciliophora with 64 OTUs (9.33%). -

Predatory Flagellates – the New Recently Discovered Deep Branches of the Eukaryotic Tree and Their Evolutionary and Ecological Significance
Protistology 14 (1), 15–22 (2020) Protistology Predatory flagellates – the new recently discovered deep branches of the eukaryotic tree and their evolutionary and ecological significance Denis V. Tikhonenkov Papanin Institute for Biology of Inland Waters, Russian Academy of Sciences, Borok, 152742, Russia | Submitted March 20, 2020 | Accepted April 6, 2020 | Summary Predatory protists are poorly studied, although they are often representing important deep-branching evolutionary lineages and new eukaryotic supergroups. This short review/opinion paper is inspired by the recent discoveries of various predatory flagellates, which form sister groups of the giant eukaryotic clusters on phylogenetic trees, and illustrate an ancestral state of one or another supergroup of eukaryotes. Here we discuss their evolutionary and ecological relevance and show that the study of such protists may be essential in addressing previously puzzling evolutionary problems, such as the origin of multicellular animals, the plastid spread trajectory, origins of photosynthesis and parasitism, evolution of mitochondrial genomes. Key words: evolution of eukaryotes, heterotrophic flagellates, mitochondrial genome, origin of animals, photosynthesis, predatory protists, tree of life Predatory flagellates and diversity of eu- of the hidden diversity of protists (Moon-van der karyotes Staay et al., 2000; López-García et al., 2001; Edg- comb et al., 2002; Massana et al., 2004; Richards The well-studied multicellular animals, plants and Bass, 2005; Tarbe et al., 2011; de Vargas et al., and fungi immediately come to mind when we hear 2015). In particular, several prevailing and very abun- the term “eukaryotes”. However, these groups of dant ribogroups such as MALV, MAST, MAOP, organisms represent a minority in the real diversity MAFO (marine alveolates, stramenopiles, opistho- of evolutionary lineages of eukaryotes. -

Protist Phylogeny and the High-Level Classification of Protozoa
Europ. J. Protistol. 39, 338–348 (2003) © Urban & Fischer Verlag http://www.urbanfischer.de/journals/ejp Protist phylogeny and the high-level classification of Protozoa Thomas Cavalier-Smith Department of Zoology, University of Oxford, South Parks Road, Oxford, OX1 3PS, UK; E-mail: [email protected] Received 1 September 2003; 29 September 2003. Accepted: 29 September 2003 Protist large-scale phylogeny is briefly reviewed and a revised higher classification of the kingdom Pro- tozoa into 11 phyla presented. Complementary gene fusions reveal a fundamental bifurcation among eu- karyotes between two major clades: the ancestrally uniciliate (often unicentriolar) unikonts and the an- cestrally biciliate bikonts, which undergo ciliary transformation by converting a younger anterior cilium into a dissimilar older posterior cilium. Unikonts comprise the ancestrally unikont protozoan phylum Amoebozoa and the opisthokonts (kingdom Animalia, phylum Choanozoa, their sisters or ancestors; and kingdom Fungi). They share a derived triple-gene fusion, absent from bikonts. Bikonts contrastingly share a derived gene fusion between dihydrofolate reductase and thymidylate synthase and include plants and all other protists, comprising the protozoan infrakingdoms Rhizaria [phyla Cercozoa and Re- taria (Radiozoa, Foraminifera)] and Excavata (phyla Loukozoa, Metamonada, Euglenozoa, Percolozoa), plus the kingdom Plantae [Viridaeplantae, Rhodophyta (sisters); Glaucophyta], the chromalveolate clade, and the protozoan phylum Apusozoa (Thecomonadea, Diphylleida). Chromalveolates comprise kingdom Chromista (Cryptista, Heterokonta, Haptophyta) and the protozoan infrakingdom Alveolata [phyla Cilio- phora and Miozoa (= Protalveolata, Dinozoa, Apicomplexa)], which diverged from a common ancestor that enslaved a red alga and evolved novel plastid protein-targeting machinery via the host rough ER and the enslaved algal plasma membrane (periplastid membrane). -
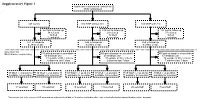
Supplementary Figure 1 Multicenter Randomised Control Trial 2746 Randomised
Supplementary Figure 1 Multicenter randomised control trial 2746 randomised 947 control 910 MNP without zinc 889 MNP with zinc 223 lost to follow up 219 lost to follow up 183 lost to follow up 34 refused 29 refused 37 refused 16 died 12 died 9 died 3 excluded 4 excluded 1 excluded 671 in follow-up 646 in follow-up 659 in follow-up at 24mo of age at 24mo of age at 24mo of age Selection for Microbiome sequencing 516 paired samples unavailable 469 paired samples unavailable 497 paired samples unavailable 69 antibiotic use 63 antibiotic use 67 antibiotic use 31 outside of WLZ criteria 37 outside of WLZ criteria 34 outside of WLZ criteria 6 diarrhea last 7 days 2 diarrhea last 7 days 7 diarrhea last 7 days 39 WLZ > -1 at 24 mo 10 WLZ < -2 at 24mo 58 WLZ > -1 at 24 mo 17 WLZ < -2 at 24mo 48 WLZ > -1 at 24 mo 8 WLZ < -2 at 24mo available for selection available for selection available for selection available for selection available for selection1 available for selection1 14 selected 10 selected 15 selected 14 selected 20 selected1 7 selected1 1 Two subjects (one in the reference WLZ group and one undernourished) had, at 12 months, no diarrhea within 1 day of stool collection but reported diarrhea within 7 days prior. Length, cm kg Weight, Supplementary Figure 2. Length (left) and weight (right) z-scores of children recruited into clinical trial NCT00705445 during the first 24 months of life. Median and quantile values are shown, with medians for participants profiled in current study indicated by red (undernourished) and black (reference WLZ) lines. -

Plasmodiophora Brassicae
Bi et al. Phytopathology Research (2019) 1:12 https://doi.org/10.1186/s42483-019-0018-6 Phytopathology Research RESEARCH Open Access Comparative genomics reveals the unique evolutionary status of Plasmodiophora brassicae and the essential role of GPCR signaling pathways Kai Bi1,2, Tao Chen2, Zhangchao He1,2, Zhixiao Gao1,2, Ying Zhao1,2, Huiquan Liu3, Yanping Fu2, Jiatao Xie1,2, Jiasen Cheng1,2 and Daohong Jiang1,2* Abstract Plasmodiophora brassicae is an important biotrophic eukaryotic plant pathogen and a member of the rhizarian protists. This biotrophic pathogen causes clubroot in cruciferous plants via novel intracellular mechanisms that are markedly different from those of other biotrophic organisms. To date, genomes from six single spore isolates of P. brassicae have been sequenced. An accurate description of the evolutionary status of this biotrophic protist, however, remains lacking. Here, we determined the draft genome of the P. brassicae ZJ-1 strain. A total of 10,951 protein-coding genes were identified from a 24.1 Mb genome sequence. We applied a comparative genomics approach to prove the Rhizaria supergroup is an independent branch in the eukaryotic evolutionary tree. We also found that the GPCR signaling pathway, the versatile signal transduction to multiple intracellular signaling cascades in response to extracellular signals in eukaryotes, is significantly enriched in P. brassicae-expanded and P. brassicae-specific gene sets. Additionally, treatment with a GPCR inhibitor relieved the symptoms of clubroot and significantly suppressed the development of plasmodia. Our findings suggest that GPCR signal transduction pathways play important roles in the growth, development, and pathogenicity of P. brassicae. -

The Revised Classification of Eukaryotes
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/231610049 The Revised Classification of Eukaryotes Article in Journal of Eukaryotic Microbiology · September 2012 DOI: 10.1111/j.1550-7408.2012.00644.x · Source: PubMed CITATIONS READS 961 2,825 25 authors, including: Sina M Adl Alastair Simpson University of Saskatchewan Dalhousie University 118 PUBLICATIONS 8,522 CITATIONS 264 PUBLICATIONS 10,739 CITATIONS SEE PROFILE SEE PROFILE Christopher E Lane David Bass University of Rhode Island Natural History Museum, London 82 PUBLICATIONS 6,233 CITATIONS 464 PUBLICATIONS 7,765 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Biodiversity and ecology of soil taste amoeba View project Predator control of diversity View project All content following this page was uploaded by Smirnov Alexey on 25 October 2017. The user has requested enhancement of the downloaded file. The Journal of Published by the International Society of Eukaryotic Microbiology Protistologists J. Eukaryot. Microbiol., 59(5), 2012 pp. 429–493 © 2012 The Author(s) Journal of Eukaryotic Microbiology © 2012 International Society of Protistologists DOI: 10.1111/j.1550-7408.2012.00644.x The Revised Classification of Eukaryotes SINA M. ADL,a,b ALASTAIR G. B. SIMPSON,b CHRISTOPHER E. LANE,c JULIUS LUKESˇ,d DAVID BASS,e SAMUEL S. BOWSER,f MATTHEW W. BROWN,g FABIEN BURKI,h MICAH DUNTHORN,i VLADIMIR HAMPL,j AARON HEISS,b MONA HOPPENRATH,k ENRIQUE LARA,l LINE LE GALL,m DENIS H. LYNN,n,1 HILARY MCMANUS,o EDWARD A. D. -

Cytoklepty in the Plankton: a Host Strategy to Optimize the Bioenergetic Machinery of Endosymbiotic Algae
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.08.416644; this version posted December 9, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Cytoklepty in the plankton: a host strategy to optimize the bioenergetic machinery of endosymbiotic algae Uwizeye Clarisse1*, Mars Brisbin Margaret2,a*, Gallet Benoit3, Chevalier Fabien1, LeKieffre Charlotte 1, Schieber L. Nicole4, Falconet Denis1, Wangpraseurt Daniel 5,6,7, Schertel Lukas 6, Stryhanyuk Hryhoriy 8, Musat Niculina 8, Mitarai Satoshi 2, Schwab Yannick 4, Finazzi Giovanni1, Decelle Johan1§ *: these authors contributed equally to the manuscript aPresent address: Biology and Marine Chemistry & Geochemistry departments, Woods Hole Oceanographic Institution, Woods Hole, USA 1- Univ. Grenoble Alpes, CNRS, CEA, INRAe, IRIG-LPCV, 38000 Grenoble, FRANCE 2- Marine Biophysics Unit, Okinawa Institute of Science and Technology, 1919-1 Tancha, Onna, Okinawa 904-0495, Japan 3-Institut de Biologie Structurale (IBS), University Grenoble Alpes, CEA, CNRS, 38044 Grenoble, France 4- Cell Biology and Biophysics Unit, European Molecular Biology Laboratory (EMBL), 69117 Heidelberg, Germany 5-Department of Nanoengineering, University of California San Diego, San Diego, CA, USA 6-Bioinspired Photonics Group, Department of Chemistry, University of Cambridge, Cambridge, UK 7-Scripps Institution of Oceanography, University of California San Diego, San Diego, USA 8-Helmholtz Centre for Environmental Research – UFZ, Department of Isotope Biogeochemistry, Leipzig, Germany §: Johan Decelle, Cell and Plant Physiology Department, 17 rue des Martyrs, 38054 Grenoble, France Email: [email protected] Keywords: symbiosis; oceanic plankton; 3D electron microscopy, photosynthesis, single-cell transcriptomics Author Contributions: C.U., M.M.B., S.M. -

Letter to the Editor
Letter to the Editor Toward the Monophyly of Haeckel's Radiolaria: 18S rRNA Environmental Data Support the Sisterhood of Polycystinea and Acantharea Puri®cacioÂn LoÂpez-GarcõÂa,*² Francisco RodrõÂguez-Valera,² and David Moreira* *Universite Pierre et Marie Curie, Paris and ²DivisioÂn de MicrobiologõÂa, Universidad Miguel HernaÂndez, San Juan de Alicante, Spain subsequently constructed a cosmid genomic library of the 0.2- to 5-mm biomass fraction from 500-m-deep wa- In 1887, Ernst Haeckel published a monumental ters at the same location (598199S, 558459W). The con- and amazingly illustrated monograph describing several struction of the environmental genomic libraries has a thousand different radiolarian species, which had been number of advantages over a direct PCR ampli®cation collected during the 4-year journey (1872±1876) of the of a target gene. First, the diversity retrieved is not af- British oceanographic corvette H.M.S. Challenger fected by well-known PCR-related biases. Second, these (Haeckel 1887). Radiolaria consist of diverse marine kinds of libraries offer the possibility of retrieving other planktonic protists, mostly unicellular, usually endowed gene sequences, in addition to the 18S rRNA, from the with complex, conspicuous mineral skeletons. Haeckel same genomic fragment. applied the term Radiolaria to three different groups: Our environmental genomic library was construct- Acantharea, Polycystinea (Spumellaria and Nassellaria), ed using 3.5 mg of DNA. DNA was mechanically bro- and Phaeodarea. All of them are united by the posses- ken by intense shearing and fractionated in a 0.8% low sion of a central capsule de®ning an intracapsular and melting point agarose gel. The band corresponding to an extracapsular region in the cytoplasm, and some of 35±45 kb DNA fragments was cut off and digested with them, (all Acantharea and several Spumellaria), also by gelase.