Red Mangrove Eradication and Pickleweed Control in a Hawaiian Wetland, Waterbird Responses, and Lessons Learned
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

'Alae 'Ula (Hawaiian Moorhen)
NATIVE WATERBIRDS AVIAN NEWCOMERS These newly-created wetlands have been rapidly colonized by native waterbirds, Many non-native birds are attracted to the wetland restoration as well. The including four species that are highly endangered and found only in the Hawaiian long-necked white waders are Cattle Egrets, native to the Old World. Non-native Islands. The ‘Alae ‘Ula, or Hawaiian Moorhen (Gallinula chloropus sandvicensis), songbirds include the Common Myna, White-rumped Shama, two unrelated kinds of and Koloa Maoli, or Koloa Duck (Anas wyvilliana), have by now raised many broods cardinals, and three kinds of doves. Many of these exotic species probably became here, nesting among the native sedges. The Ae‘o, or Hawaiian Stilt (Himantopus established in recent decades as escaped cage birds. Before the accidental mexicanus knudseni), and the Nēnē, or Hawaiian Goose (Branta sandvicensis), stop introduction of mosquitoes in the 19th century and bird diseases they carry, by almost daily to rest and feed. In the morning and evening, watch for the ‘Auku‘u these coastal lowlands were home to native honeycreepers and other native or Black-crowned Night Heron (Nycticorax nycticorax). Long-distance migrants such songbirds, preserved abundantly in the fossil record of Makauwahi Cave. as the Kōlea or Pacific Golden Plover Pluvialis( fulva) stop to rest and often winter here, as part of their annual 10,000-mile migration from breeding grounds in the Arctic to wintering sites in the tropics. Bones of all these bird species occur as fossils in the sediment of adjacent Makauwahi Cave, showing that they have thrived here for thousands of years. -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Growth Patterns of Hawaiian Stilt Chicks
Wilson Bull., 11 l(4), 1999, pp. 478487 GROWTH PATTERNS OF HAWAIIAN STILT CHICKS J. MICHAEL REED,,2,8‘ ELIZABETH M. GRAY,334 DIANNE LEWIS3 LEWIS W. ORING,3 RICHARD COLEMAN,5 TIMOTHY BURR,6 AND PETER LUSCOMB7 ABSTRACT-We studied chick growth and plumage patterns in the endangered Hawaiian Stilt (Himantopus mexicanus knudseni). Body mass of captive chicks closely fit a Gompertz growth curve, revealing a growth coefficient (K) of 0.065 day- ’ and point of inflection (T) of 17 days. When chicks fledged about 28 days after hatching, they weighed only 60% of adult body mass; at 42 d, birds still were only 75% of adult mass; culmen, tarsus, and wing chord at fledging also were less than adult size. This trend of continued growth to adult size after fledging is typical for most shorebirds. After hatching, captive chicks grew more rapidly than wild chicks, probably because of an unlimited food supply. We found no evidence for adverse effects of weather on the growth of wild chicks. As with other shorebirds, the tarsus started relatively long, with culmen and then wing chord growing more rapidly in later development. Tarsal and wing chord growth were sigmoidal, whereas culmen growth was linear. We describe plumage characteristics of weekly age classes of chicks to help researchers age birds in the wild. Received 28 Dec. 1998, accepted 20 April 1999. Avian growth patterns have been studied (Himantopus mexicanus knudseni), a precocial primarily because of their relationships to the bird that is an endangered subspecies of the ecology and evolutionary history of different Black-necked Stilt. -
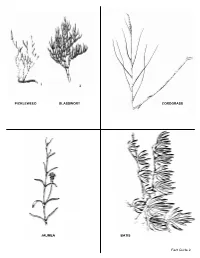
Plant Field Guide
2 PICKLEWEED GLASSWORT CORDGRASS JAUMEA BATIS Field Guide 9 PICKLEWEED Amaranth Family 3 kinds, 2 examples CORDGRASS Grass Family 1 Pickleweed Sarcocornia pacifica Spartina foliosa Glasswort Arthrocnemum subterminalis 2 HABITAT: Growns in the low marsh where the HABITAT: Found throughout the salt marsh. roots are continually bathed in ocean water. APPEARANCE: Stems look like a chain of small APPEARANCE: Look for a tall grass which is pickles. higher than the other plants in the salt marsh. REPRODUCTION: The flowers of all pickleweeds REPRODUCTION: All grasses are wind pollinated. are pollinated by the wind. The small flowers are Look for straw colored spikes of densely packed hard to see because they have no colorful petals flowers. Male flowers will have pollen and the female flowers will show graceful waving stigmas to ADAPTATION TO SALT: Pickleweeds are some of catch the pollen. the many marsh plants that use salt storage (they are accumulators). Also called succulents, these ADAPTATION TO SALT: All the salt marsh plants are swollen with the stored salty water. grasses are salt excreters using special pores to When the salt concentration becomes too high the push out droplets of salty water. Look on the grass cells will die. blades for salt crystals. See sea lavender. ECOLOGICAL RELATIONSHIPS: Frequently the ECOLOGICAL RELATIONSHIPS: Home for the most common plants in the marsh, they provide endangered bird, the Light-footed Clapper Rail. shelter and food for invertebrates. Belding’s A spider lives its whole life inside the blades. Savannah Sparrows build their nests in the Important food for grazing animals. glasswort. BATIS or SALTWORT Saltwort Family Batis maritima HABITAT: Most frequently found in the low marsh. -

Maritime Swamp Forest (Typic Subtype)
MARITIME SWAMP FOREST (TYPIC SUBTYPE) Concept: Maritime Swamp Forests are wetland forests of barrier islands and comparable coastal spits and back-barrier islands, dominated by tall trees of various species. The Typic Subtype includes most examples, which are not dominated by Acer, Nyssa, or Fraxinus, not by Taxodium distichum. Canopy dominants are quite variable among the few examples. Distinguishing Features: Maritime Shrub Swamps are distinguished from other barrier island wetlands by dominance by tree species of (at least potentially) large stature. The Typic Subtype is dominated by combinations of Nyssa, Fraxinus, Liquidambar, Acer, or Quercus nigra, rather than by Taxodium or Salix. Maritime Shrub Swamps are dominated by tall shrubs or small trees, particularly Salix, Persea, or wetland Cornus. Some portions of Maritime Evergreen Forest are marginally wet, but such areas are distinguished by the characteristic canopy dominants of that type, such as Quercus virginiana, Quercus hemisphaerica, or Pinus taeda. The lower strata also are distinctive, with wetland species occurring in Maritime Swamp Forest; however, some species, such as Morella cerifera, may occur in both. Synonyms: Acer rubrum - Nyssa biflora - (Liquidambar styraciflua, Fraxinus sp.) Maritime Swamp Forest (CEGL004082). Ecological Systems: Central Atlantic Coastal Plain Maritime Forest (CES203.261). Sites: Maritime Swamp Forests occur on barrier islands and comparable spits, in well-protected dune swales, edges of dune ridges, and on flats adjacent to freshwater sounds. Soils: Soils are wet sands or mucky sands, most often mapped as Duckston (Typic Psammaquent) or Conaby (Histic Humaquept). Hydrology: Most Maritime Swamp Forests have shallow seasonal standing water and nearly permanently saturated soils. Some may rarely be flooded by salt water during severe storms, but areas that are severely or repeatedly flooded do not recover to swamp forest. -

PESHTIGO RIVER DELTA Property Owner
NORTHEAST - 10 PESHTIGO RIVER DELTA WETLAND TYPES Drew Feldkirchner Floodplain forest, lowland hardwood, swamp, sedge meadow, marsh, shrub carr ECOLOGY & SIGNIFICANCE supports cordgrass, marsh fern, sensitive fern, northern tickseed sunflower, spotted joe-pye weed, orange This Wetland Gem site comprises a very large coastal • jewelweed, turtlehead, marsh cinquefoil, blue skullcap wetland complex along the northwest shore of Green Bay and marsh bellflower. Shrub carr habitat is dominated three miles southeast of the city of Peshtigo. The wetland by slender willow; other shrub species include alder, complex extends upstream along the Peshtigo River for MARINETTE COUNTY red osier dogwood and white meadowsweet. Floodplain two miles from its mouth. This site is significant because forest habitats are dominated by silver maple and green of its size, the diversity of wetland community types ash. Wetlands of the Peshtigo River Delta support several present, and the overall good condition of the vegetation. - rare plant species including few-flowered spikerush, The complexity of the site – including abandoned oxbow variegated horsetail and northern wild raisin. lakes and a series of sloughs and lagoons within the river delta – offers excellent habitat for waterfowl. A number This Wetland Gem provides extensive, diverse and high of rare animals and plants have been documented using quality wetland habitat for many species of waterfowl, these wetlands. The area supports a variety of recreational herons, gulls, terns and shorebirds and is an important uses, such as hunting, fishing, trapping and boating. The staging, nesting and stopover site for many migratory Peshtigo River Delta has been described as the most birds. Rare and interesting bird species documented at diverse and least disturbed wetland complex on the west the site include red-shouldered hawk, black tern, yellow shore of Green Bay. -

National Wildlife Refuges Changed2.Pub
TAKE REFUGE Celebrating 100 Years of Threatened and Endangered Species Protection Through the National Wildlife Refuge System TAKE REFUGE Celebrating 100 Years of Threatened and Endangered Species Protection Through the National Wildlife Refuge System The State Public Interest Research Groups U.S. PIRG Education Fund March 2003 Written and designed by: Shannon Ryan, U.S. PIRG Education Fund For more information: Shannon Ryan U.S. Public Interest Research Group Education Fund 218 D Street, SE, Washington, DC 20003 Copies of this report may be ordered by sending a check or money order for $35.00 to: U.S. PIRG, 218 D Street, SE, Washington, DC 20003 The author would like to thank the following people: Alison Cassady and Tiernan Sittenfeld for research and writing assistance; Alicia Supernavage for production assistance; and refuge staff at Sauta Cave NWR, Buenos Aires NWR, Don Edwards San Francisco Bay NWR, Archie Carr NWR, Ash Meadows NWR, Nestucca Bay NWR, Attwater Prairie Chicken NWR, and James River NWR for their time and expertise. Production of this report would not have been possible without funding from: Center for Biological Diversity Defenders of Wildlife National Wildlife Federation Sierra Club PHOTOGRAPHY CREDITS: Pages 11 & 12: Turtle tracks, Archie Carr NWR (background), Friends of the Carr Refuge. Cover & Back Cover: Lange’s metalmark butterfly, USFWS/David Wright; salt marsh bird’s beak, ©Thomas Oberbauer; sandhill crane, USFWS/D.D. Iwurst; Pages 13 & 14: Crystal Spring, Ash Meadows NWR (background), The Mason Neck NWR (background), Mason Neck Canoe and Kayak. American Southwest. Acknowledgements & Table of Contents: Balcones Canyonlands NWR Pages 15 & 16: Nestucca Bay NWR (background), USFWS/David Pitkin. -

Tidal Wetland Gross Primary Production Across the Continental
RESEARCH ARTICLE Tidal Wetland Gross Primary Production Across 10.1029/2019GB006349 the Continental United States, 2000–2019 Special Section: R. A. Feagin1 , I. Forbrich2 , T. P. Huff1, J. G. Barr3, J. Ruiz‐Plancarte4 , J. D. Fuentes4 , Carbon cycling in tidal wet- 4 5 5 6 7 R. G. Najjar , R. Vargas , A. Vázquez‐Lule , L. Windham‐Myers , K. D. Kroeger , lands and estuaries of the con- 8 1 9 8 8 2 tiguous United States E. J. Ward , G. W. Moore , M. Leclerc , K. W. Krauss , C. L. Stagg , M. Alber , S. H. Knox10 , K. V. R. Schäfer11, T. S. Bianchi12 , J. A. Hutchings13 , H. Nahrawi9,14 , 1 1 1 1,15 6 16 Key Points: A. Noormets , B. Mitra , A. Jaimes , A. L. Hinson , B. Bergamaschi , J. S. King , and 17 • We created the Blue Carbon (BC) G. Miao model, which mapped the Gross Primary Production (GPP) of all 1Department of Ecology and Conservation Biology, Texas A&M University, College Station, TX, USA, 2Marine Biological tidal wetlands within the Laboratory, Woods Hole, MA, USA, 3Elder Research, Charlottesville, VA, USA, 4Department of Meteorology and continental United States 5 • Atmospheric Science, Pennsylvania State University, University Park, PA, USA, Department of Plant and Soil Sciences, The BC model provides maps of tidal 6 7 wetland GPP at sub‐250 m scales University of Delaware, Newark, DE, USA, United States Geological Survey, Menlo Park, CA, USA, United States 8 and at 16‐day intervals for the years Geological Survey, Woods Hole, MA, USA, United States Geological Survey, Wetland and Aquatic Research Center, 2000‐2019 Lafayette, LA, USA, 9Atmospheric Biogeosciences, University of Georgia, Athens, GA, USA, 10Department of Geography, • 2 The average daily GPP per m was University of British Columbia, Vancouver, BC, USA, 11Department of Biological Sciences, Rutgers University, Newark, 4.32 ± 2.45 g C/m2/day, and the total NJ, USA, 12Departmet of Geological Sciences, University of Florida, Gainesville, FL, USA, 13Department of Earth and annual GPP for the continental 14 United States was 39.65 ± 0.89 Tg Planetary Sciences, Washington University, St. -

Exploring Our Wonderful Wetlands Publication
Exploring Our Wonderful Wetlands Student Publication Grades 4–7 Dear Wetland Students: Are you ready to explore our wonderful wetlands? We hope so! To help you learn about several types of wetlands in our area, we are taking you on a series of explorations. As you move through the publication, be sure to test your wetland wit and write about wetlands before moving on to the next exploration. By exploring our wonderful wetlands, we hope that you will appreciate where you live and encourage others to help protect our precious natural resources. Let’s begin our exploration now! Southwest Florida Water Management District Exploring Our Wonderful Wetlands Exploration 1 Wading Into Our Wetlands ................................................Page 3 Exploration 2 Searching Our Saltwater Wetlands .................................Page 5 Exploration 3 Finding Out About Our Freshwater Wetlands .............Page 7 Exploration 4 Discovering What Wetlands Do .................................... Page 10 Exploration 5 Becoming Protectors of Our Wetlands ........................Page 14 Wetlands Activities .............................................................Page 17 Websites ................................................................................Page 20 Visit the Southwest Florida Water Management District’s website at WaterMatters.org. Exploration 1 Wading Into Our Wetlands What exactly is a wetland? The scientific and legal definitions of wetlands differ. In 1984, when the Florida Legislature passed a Wetlands Protection Act, they decided to use a plant list containing plants usually found in wetlands. We are very fortunate to have a lot of wetlands in Florida. In fact, Florida has the third largest wetland acreage in the United States. The term wetlands includes a wide variety of aquatic habitats. Wetland ecosystems include swamps, marshes, wet meadows, bogs and fens. Essentially, wetlands are transitional areas between dry uplands and aquatic systems such as lakes, rivers or oceans. -

A Habitat Conservation Plan for Hawaiian Stilt at Cyanotech Corporation
A Habitat Conservation Plan for Hawaiian Stilt at Cyanotech Corporation. Keahole Point, Hawaii. March 2006 through March 2016 April 2006 Prepared by Cyanotech Corporation Kailua-Kona, Hawaii EXECUTIVE SUMMARY Cyanotech Corporation (Cyanotech) has applied for a permit from the U.S. Fish and Wildlife Service (USFWS) pursuant to section 10(a)(l)(B) of the Endangered Species Act of 1973 (ESA) (16 U.S.C. 1531-1544), as amended, and has applied for a license from the Hawaii Department of Land and Natural Resources (HDLNR) in accordance with the HRS (Hawaii Revised Statutes) section 195D-4(g) to incidentally take endangered Hawaiian Stilt (Himantopus mexicanus knudseni). The incidental take is anticipated to occur as a result of ongoing operations and maintenance activities at Cyanotech’s aquaculture facility within the Natural Energy Laboratory of Hawaii (NELHA) along the Kona Coast of the island of Hawaii (Big Island). No other listed, proposed, or candidate species are found in the project area. In support of the permit application, Cyanotech proposes to implement a Conservation Plan as required by section 10(a)(2)(A) of the ESA and the HRS section 195D-21. The proposed permit period is ten years. The primary goal of the Conservation Plan for Hawaiian Stilt at Cyanotech is to eliminate the incidental take of Hawaiian Stilt by eliminating the “attractive nuisance” problem created by the expanse of open-water ponds, invertebrate food resources, and remote nesting areas, which inadvertently attract Hawaiian Stilt to the Cyanotech facility. The purpose of the Conservation Plan is to actively pursue non-lethal bird deterrent measures to reduce and eliminate stilt foraging and nesting at the facility. -

List of Shorebird Profiles
List of Shorebird Profiles Pacific Central Atlantic Species Page Flyway Flyway Flyway American Oystercatcher (Haematopus palliatus) •513 American Avocet (Recurvirostra americana) •••499 Black-bellied Plover (Pluvialis squatarola) •488 Black-necked Stilt (Himantopus mexicanus) •••501 Black Oystercatcher (Haematopus bachmani)•490 Buff-breasted Sandpiper (Tryngites subruficollis) •511 Dowitcher (Limnodromus spp.)•••485 Dunlin (Calidris alpina)•••483 Hudsonian Godwit (Limosa haemestica)••475 Killdeer (Charadrius vociferus)•••492 Long-billed Curlew (Numenius americanus) ••503 Marbled Godwit (Limosa fedoa)••505 Pacific Golden-Plover (Pluvialis fulva) •497 Red Knot (Calidris canutus rufa)••473 Ruddy Turnstone (Arenaria interpres)•••479 Sanderling (Calidris alba)•••477 Snowy Plover (Charadrius alexandrinus)••494 Spotted Sandpiper (Actitis macularia)•••507 Upland Sandpiper (Bartramia longicauda)•509 Western Sandpiper (Calidris mauri) •••481 Wilson’s Phalarope (Phalaropus tricolor) ••515 All illustrations in these profiles are copyrighted © George C. West, and used with permission. To view his work go to http://www.birchwoodstudio.com. S H O R E B I R D S M 472 I Explore the World with Shorebirds! S A T R ER G S RO CHOOLS P Red Knot (Calidris canutus) Description The Red Knot is a chunky, medium sized shorebird that measures about 10 inches from bill to tail. When in its breeding plumage, the edges of its head and the underside of its neck and belly are orangish. The bird’s upper body is streaked a dark brown. It has a brownish gray tail and yellow green legs and feet. In the winter, the Red Knot carries a plain, grayish plumage that has very few distinctive features. Call Its call is a low, two-note whistle that sometimes includes a churring “knot” sound that is what inspired its name. -

Coastal Wetland Trends in the Narragansett Bay Estuary During the 20Th Century
u.s. Fish and Wildlife Service Co l\Ietland Trends In the Narragansett Bay Estuary During the 20th Century Coastal Wetland Trends in the Narragansett Bay Estuary During the 20th Century November 2004 A National Wetlands Inventory Cooperative Interagency Report Coastal Wetland Trends in the Narragansett Bay Estuary During the 20th Century Ralph W. Tiner1, Irene J. Huber2, Todd Nuerminger2, and Aimée L. Mandeville3 1U.S. Fish & Wildlife Service National Wetlands Inventory Program Northeast Region 300 Westgate Center Drive Hadley, MA 01035 2Natural Resources Assessment Group Department of Plant and Soil Sciences University of Massachusetts Stockbridge Hall Amherst, MA 01003 3Department of Natural Resources Science Environmental Data Center University of Rhode Island 1 Greenhouse Road, Room 105 Kingston, RI 02881 November 2004 National Wetlands Inventory Cooperative Interagency Report between U.S. Fish & Wildlife Service, University of Massachusetts-Amherst, University of Rhode Island, and Rhode Island Department of Environmental Management This report should be cited as: Tiner, R.W., I.J. Huber, T. Nuerminger, and A.L. Mandeville. 2004. Coastal Wetland Trends in the Narragansett Bay Estuary During the 20th Century. U.S. Fish and Wildlife Service, Northeast Region, Hadley, MA. In cooperation with the University of Massachusetts-Amherst and the University of Rhode Island. National Wetlands Inventory Cooperative Interagency Report. 37 pp. plus appendices. Table of Contents Page Introduction 1 Study Area 1 Methods 5 Data Compilation 5 Geospatial Database Construction and GIS Analysis 8 Results 9 Baywide 1996 Status 9 Coastal Wetlands and Waters 9 500-foot Buffer Zone 9 Baywide Trends 1951/2 to 1996 15 Coastal Wetland Trends 15 500-foot Buffer Zone Around Coastal Wetlands 15 Trends for Pilot Study Areas 25 Conclusions 35 Acknowledgments 36 References 37 Appendices A.