Patterns of Feeding Behaviour in Adult Male Riodinid Butterflies and Their
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Lepidoptera: Papilionoidea) Q ⇑ Marianne Espeland A,B, , Jason P.W
Molecular Phylogenetics and Evolution 93 (2015) 296–306 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Ancient Neotropical origin and recent recolonisation: Phylogeny, biogeography and diversification of the Riodinidae (Lepidoptera: Papilionoidea) q ⇑ Marianne Espeland a,b, , Jason P.W. Hall c, Philip J. DeVries d, David C. Lees e, Mark Cornwall a, Yu-Feng Hsu f, Li-Wei Wu g, Dana L. Campbell a,h, Gerard Talavera a,i,j, Roger Vila i, Shayla Salzman a, Sophie Ruehr k, David J. Lohman l, Naomi E. Pierce a a Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, 26 Oxford Street, Cambridge, MA 02138, USA b McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Powell Hall, 2315 Hull Road, Gainesville, FL 32611, USA c Department of Systematic Biology-Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560-127, USA d Department of Biological Sciences, University of New Orleans, 2000 Lake Shore Drive, New Orleans, LA 70148, USA e Department of Zoology, University of Cambridge, Cambridge CB2 3EJ, UK f Department of Life Science, National Taiwan Normal University, Taipei, Taiwan g The Experimental Forest, College of Bio-Resources and Agriculture, National Taiwan University, Nantou, Taiwan h Division of Biological Sciences, School of Science, Technology, Engineering & Mathematics, University of Washington Bothell, Box 358500, 18115 Campus Way NE, Bothell, WA 98011-8246, USA i Institut de Biologia Evolutiva (CSIC-UPF), Pg. Marítim de la Barceloneta 37, 08003 Barcelona, Spain j Faculty of Biology & Soil Science, St. -

Lepidoptera, Papilionoidea) in a Heterogeneous Area Between Two Biodiversity Hotspots in Minas Gerais, Brazil
ARTICLE Butterfly fauna (Lepidoptera, Papilionoidea) in a heterogeneous area between two biodiversity hotspots in Minas Gerais, Brazil Déborah Soldati¹³; Fernando Amaral da Silveira¹⁴ & André Roberto Melo Silva² ¹ Universidade Federal de Minas Gerais (UFMG), Instituto de Ciências Biológicas (ICB), Departamento de Zoologia, Laboratório de Sistemática de Insetos. Belo Horizonte, MG, Brasil. ² Centro Universitário UNA, Faculdade de Ciências Biológicas e da Saúde. Belo Horizonte, MG, Brasil. ORCID: http://orcid.org/0000-0003-3113-5840. E-mail: [email protected] ³ ORCID: http://orcid.org/0000-0002-9546-2376. E-mail: [email protected] (corresponding author). ⁴ ORCID: http://orcid.org/0000-0003-2408-2656. E-mail: [email protected] Abstract. This paper investigates the butterfly fauna of the ‘Serra do Rola-Moça’ State Park, Minas Gerais, Brazil. We eval- uate i) the seasonal variation of species richness and composition; and ii) the variation in composition of the local butterfly assemblage among three sampling sites and between the dry and rainy seasons. Sampling was carried out monthly between November 2012 and October 2013, using entomological nets. After a total sampling effort of 504 net hours, 311 species were recorded. One of them is endangered in Brazil, and eight are probable new species. Furthermore, two species were new records for the region and eight considered endemic of the Cerrado domain. There was no significant difference in species richness between the dry and the rainy seasons, however the species composition varies significantly among sampling sites. Due to its special, heterogeneous environment, which is home to a rich butterfly fauna, its preservation is important for the conservation of the regional butterfly fauna. -

North Andean Origin and Diversification of the Largest Ithomiine Butterfly Genus
North Andean origin and diversification of the largest ithomiine butterfly genus The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Lisa De-Silva, D., L. L. Mota, N. Chazot, R. Mallarino, K. L. Silva- Brandão, L. M. G. Piñerez, A. V. Freitas, et al. 2017. “North Andean origin and diversification of the largest ithomiine butterfly genus.” Scientific Reports 7 (1): 45966. doi:10.1038/srep45966. http:// dx.doi.org/10.1038/srep45966. Published Version doi:10.1038/srep45966 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:32630680 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA www.nature.com/scientificreports OPEN North Andean origin and diversification of the largest ithomiine butterfly genus Received: 31 October 2016 Donna Lisa De-Silva1, Luísa L. Mota2, Nicolas Chazot1,3, Ricardo Mallarino4, Karina L. Silva- Accepted: 22 February 2017 Brandão5, Luz Miryam Gómez Piñerez6,7, André V.L. Freitas2, Gerardo Lamas8, Published: 07 April 2017 Mathieu Joron9, James Mallet4, Carlos E. Giraldo6,10, Sandra Uribe6, Tiina Särkinen11, Sandra Knapp12, Chris D. Jiggins13, Keith R. Willmott14 & Marianne Elias1 The Neotropics harbour the most diverse flora and fauna on Earth. The Andes are a major centre of diversification and source of diversity for adjacent areas in plants and vertebrates, but studies on insects remain scarce, even though they constitute the largest fraction of terrestrial biodiversity. -

Bulletin Biological Assessment Boletín RAP Evaluación Biológica
Rapid Assessment Program Programa de Evaluación Rápida Evaluación Biológica Rápida de Chawi Grande, Comunidad Huaylipaya, Zongo, La Paz, Bolivia RAP Bulletin A Rapid Biological Assessment of of Biological Chawi Grande, Comunidad Huaylipaya, Assessment Zongo, La Paz, Bolivia Boletín RAP de Evaluación Editores/Editors Biológica Claudia F. Cortez F., Trond H. Larsen, Eduardo Forno y Juan Carlos Ledezma 70 Conservación Internacional Museo Nacional de Historia Natural Gobierno Autónomo Municipal de La Paz Rapid Assessment Program Programa de Evaluación Rápida Evaluación Biológica Rápida de Chawi Grande, Comunidad Huaylipaya, Zongo, La Paz, Bolivia RAP Bulletin A Rapid Biological Assessment of of Biological Chawi Grande, Comunidad Huaylipaya, Assessment Zongo, La Paz, Bolivia Boletín RAP de Evaluación Editores/Editors Biológica Claudia F. Cortez F., Trond H. Larsen, Eduardo Forno y Juan Carlos Ledezma 70 Conservación Internacional Museo Nacional de Historia Natural Gobierno Autónomo Municipal de La Paz The RAP Bulletin of Biological Assessment is published by: Conservation International 2011 Crystal Drive, Suite 500 Arlington, VA USA 22202 Tel: +1 703-341-2400 www.conservation.org Cover Photos: Trond H. Larsen (Chironius scurrulus). Editors: Claudia F. Cortez F., Trond H. Larsen, Eduardo Forno y Juan Carlos Ledezma Design: Jaime Fernando Mercado Murillo Map: Juan Carlos Ledezma y Veronica Castillo ISBN 978-1-948495-00-4 ©2018 Conservation International All rights reserved. Conservation International is a private, non-proft organization exempt from federal income tax under section 501c(3) of the Internal Revenue Code. The designations of geographical entities in this publication, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of Conservation International or its supporting organizations concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Developmental, Cellular and Biochemical Basis of Transparency in Clearwing Butterflies Aaron F
© 2021. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2021) 224, jeb237917. doi:10.1242/jeb.237917 RESEARCH ARTICLE Developmental, cellular and biochemical basis of transparency in clearwing butterflies Aaron F. Pomerantz1,2,*, Radwanul H. Siddique3,4, Elizabeth I. Cash5, Yuriko Kishi6,7, Charline Pinna8, Kasia Hammar2, Doris Gomez9, Marianne Elias8 and Nipam H. Patel1,2,6,* ABSTRACT INTRODUCTION The wings of butterflies and moths (Lepidoptera) are typically covered The wings of butterflies and moths (Lepidoptera) have inspired with thousands of flat, overlapping scales that endow the wings with studies across a variety of scientific fields, including evolutionary colorful patterns. Yet, numerous species of Lepidoptera have evolved biology, ecology and biophysics (Beldade and Brakefield, 2002; highly transparent wings, which often possess scales of altered Prum et al., 2006; Gilbert and Singer, 1975). Lepidopteran wings morphology and reduced size, and the presence of membrane are generally covered with rows of flat, partially overlapping surface nanostructures that dramatically reduce reflection. Optical scales that endow the wings with colorful patterns. Adult scales are properties and anti-reflective nanostructures have been characterized chitin-covered projections that serve as the unit of color for the wing. for several ‘clearwing’ Lepidoptera, but the developmental processes Each scale can generate color through pigmentation via molecules underlying wing transparency are unknown. Here, we applied that selectively absorb certain wavelengths of light, structural confocal and electron microscopy to create a developmental time coloration, which results from light interacting with the physical series in the glasswing butterfly, Greta oto, comparing transparent nanoarchitecture of the scale; or a combination of both pigmentary and non-transparent wing regions. -

Genomic Analysis of the Tribe Emesidini (Lepidoptera: Riodinidae)
Zootaxa 4668 (4): 475–488 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2019 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4668.4.2 http://zoobank.org/urn:lsid:zoobank.org:pub:211AFB6A-8C0A-4AB2-8CF6-981E12C24934 Genomic analysis of the tribe Emesidini (Lepidoptera: Riodinidae) JING ZHANG1, JINHUI SHEN1, QIAN CONG1,2 & NICK V. GRISHIN1,3 1Departments of Biophysics and Biochemistry, University of Texas Southwestern Medical Center, and 3Howard Hughes Medical Insti- tute, 5323 Harry Hines Blvd, Dallas, TX, USA 75390-9050; [email protected] 2present address: Institute for Protein Design and Department of Biochemistry, University of Washington, 1959 NE Pacific Street, HSB J-405, Seattle, WA, USA 98195; [email protected] Abstract We obtained and phylogenetically analyzed whole genome shotgun sequences of nearly all species from the tribe Emesidini Seraphim, Freitas & Kaminski, 2018 (Riodinidae) and representatives from other Riodinidae tribes. We see that the recently proposed genera Neoapodemia Trujano, 2018 and Plesioarida Trujano & García, 2018 are closely allied with Apodemia C. & R. Felder, [1865] and are better viewed as its subgenera, new status. Overall, Emesis Fabricius, 1807 and Apodemia (even after inclusion of the two subgenera) are so phylogenetically close that several species have been previously swapped between these two genera. New combinations are: Apodemia (Neoapodemia) zela (Butler, 1870), Apodemia (Neoapodemia) ares (Edwards, 1882), and Apodemia (Neoapodemia) arnacis (Stichel, 1928) (not Emesis); and Emesis phyciodoides (Barnes & Benjamin, 1924) (not Apodemia), assigned to each genus by their monophyly in genomic trees with the type species (TS) of the genus. -

A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1967 A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Ross, Gary Noel, "A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico." (1967). LSU Historical Dissertations and Theses. 1315. https://digitalcommons.lsu.edu/gradschool_disstheses/1315 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 67-14,010 ROSS, Gary Noel, 1940- A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO. Louisiana State University and Agricultural and Mechanical CoUege, Ph.D., 1967 Entomology University Microfilms, Inc., Ann Arbor, Michigan A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO A D issertation Submitted to the Graduate Faculty of the Louisiana State University and A gricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Gary Noel Ross M.S., Louisiana State University, 196*+ May, 1967 FRONTISPIECE Section of the south wall of the crater of Volcan Santa Marta. May 1965, 5,100 feet. ACKNOWLEDGMENTS Many persons have contributed to and assisted me in the prep aration of this dissertation and I wish to express my sincerest ap preciation to them all. -

Butterflies from the Middle Eocene: the Earliest Occurrence of Fossil Papilionoidea (Lepidoptera)
THE PEARCE- SELLARDS Sctks NUMBER 29 BUTTERFLIES FROM THE MIDDLE EOCENE: THE EARLIEST OCCURRENCE OF FOSSIL PAPILIONOIDEA (LEPIDOPTERA) Christopher J. Durden and Hugh Rose 1978 Texas Memorial Museum/2400 Trinity/Austin, Texas 78705 W. W. Newcomb, Director The Pearce-Sellards Series is an occasional, miscellaneous series of brief reports of museum and museum associated field investigations and other research. Its title seeks to commemorate the first two directors of the Texas Memorial Museum, now both deceased: J. E. Pearce and Dr. E. H. Sellards, professors of anthropology and geology respectively, of The University of Texas. A complete list of Pearce-Sellards papers, as well as other publica- tions of the museum, will be sent upon request. BUTTERFLIES FROM THE MIDDLE EOCENE: THE EARLIEST OCCURRENCE OF FOSSIL PAPILIONOIDEA (LEPIDOPTERA) 1 Christopher J. Durden 2 and Hugh Rose 3 ABSTRACT Three fossil butterflies recently collected from the Green River Shale of Colorado extend the known range of Rhopalocera eight to ten million years back, to 48 Ma. Praepapilio Colorado n. g., n. sp., and P. gracilis n. sp. are primitive Papilionidae related to the modern Baronia brevicornis Salvin, but they require a new subfamily, Praepapilioninae. Riodinella nympha n. g., n. sp. is a primitive member of the Lycaenidae, related to modern Ancyluris, Riodina, and Rhetus, in the tribe Riodinidi. INTRODUCTION With approximately 194,000 living species, the Lepidoptera is, after the Coleoptera with some 350,000, species, the second most diverse order of organisms. It is underrepresented in the fossil record (Scudder 1875, 1891, 1892; Handlirsch 1925;Mackay 1970;Kuhne 1973; Shields 1976). -

INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a Synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a Historical Sketch
ZOOLOGÍA-TAXONOMÍA www.unal.edu.co/icn/publicaciones/caldasia.htm Caldasia 31(2):407-440. 2009 HACIA UNA SÍNTESIS DE LOS PAPILIONOIDEA (INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a historical sketch JOSÉ LUIS SALINAS-GUTIÉRREZ El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] CLAUDIO MÉNDEZ Escuela de Biología, Universidad de San Carlos, Ciudad Universitaria, Campus Central USAC, Zona 12. Guatemala, Guatemala. [email protected] MERCEDES BARRIOS Centro de Estudios Conservacionistas (CECON), Universidad de San Carlos, Avenida La Reforma 0-53, Zona 10, Guatemala, Guatemala. [email protected] CARMEN POZO El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] JORGE LLORENTE-BOUSQUETS Museo de Zoología, Facultad de Ciencias, UNAM. Apartado Postal 70-399, México D.F. 04510; México. [email protected]. Autor responsable. RESUMEN La riqueza biológica de Mesoamérica es enorme. Dentro de esta gran área geográfi ca se encuentran algunos de los ecosistemas más diversos del planeta (selvas tropicales), así como varios de los principales centros de endemismo en el mundo (bosques nublados). Países como Guatemala, en esta gran área biogeográfi ca, tiene grandes zonas de bosque húmedo tropical y bosque mesófi lo, por esta razón es muy importante para analizar la diversidad en la región. Lamentablemente, la fauna de mariposas de Guatemala es poco conocida y por lo tanto, es necesario llevar a cabo un estudio y análisis de la composición y la diversidad de las mariposas (Lepidoptera: Papilionoidea) en Guatemala. -

Butterflies (Lepidoptera: Papilionoidea) in a Coastal Plain Area in the State of Paraná, Brazil
62 TROP. LEPID. RES., 26(2): 62-67, 2016 LEVISKI ET AL.: Butterflies in Paraná Butterflies (Lepidoptera: Papilionoidea) in a coastal plain area in the state of Paraná, Brazil Gabriela Lourenço Leviski¹*, Luziany Queiroz-Santos¹, Ricardo Russo Siewert¹, Lucy Mila Garcia Salik¹, Mirna Martins Casagrande¹ and Olaf Hermann Hendrik Mielke¹ ¹ Laboratório de Estudos de Lepidoptera Neotropical, Departamento de Zoologia, Universidade Federal do Paraná, Caixa Postal 19.020, 81.531-980, Curitiba, Paraná, Brazil Corresponding author: E-mail: [email protected]٭ Abstract: The coastal plain environments of southern Brazil are neglected and poorly represented in Conservation Units. In view of the importance of sampling these areas, the present study conducted the first butterfly inventory of a coastal area in the state of Paraná. Samples were taken in the Floresta Estadual do Palmito, from February 2014 through January 2015, using insect nets and traps for fruit-feeding butterfly species. A total of 200 species were recorded, in the families Hesperiidae (77), Nymphalidae (73), Riodinidae (20), Lycaenidae (19), Pieridae (7) and Papilionidae (4). Particularly notable records included the rare and vulnerable Pseudotinea hemis (Schaus, 1927), representing the lowest elevation record for this species, and Temenis huebneri korallion Fruhstorfer, 1912, a new record for Paraná. These results reinforce the need to direct sampling efforts to poorly inventoried areas, to increase knowledge of the distribution and occurrence patterns of butterflies in Brazil. Key words: Atlantic Forest, Biodiversity, conservation, inventory, species richness. INTRODUCTION the importance of inventories to knowledge of the fauna and its conservation, the present study inventoried the species of Faunal inventories are important for providing knowledge butterflies of the Floresta Estadual do Palmito. -

Developmental, Cellular, and Biochemical
Developmental, cellular, and biochemical basis of transparency in the glasswing butterfly Greta oto Aaron Pomerantz, Radwanul Siddique, Elizabeth Cash, Yuriko Kishi, Charline Pinna, Kasia Hammar, Doris Gomez, Marianne Elias, Nipam Patel To cite this version: Aaron Pomerantz, Radwanul Siddique, Elizabeth Cash, Yuriko Kishi, Charline Pinna, et al.. Devel- opmental, cellular, and biochemical basis of transparency in the glasswing butterfly Greta oto. 2020. hal-03012452 HAL Id: hal-03012452 https://hal.archives-ouvertes.fr/hal-03012452 Preprint submitted on 18 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. bioRxiv preprint doi: https://doi.org/10.1101/2020.07.02.183590; this version posted July 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Title 2 Developmental, cellular, and biochemical basis of transparency in the glasswing butterfly 3 Greta oto 4 5 Authors 6 Aaron F. Pomerantz1,2*, Radwanul H. Siddique3,4, Elizabeth I. Cash5, Yuriko Kishi6,7, 7 Charline Pinna8, Kasia Hammar2, Doris Gomez9, Marianne Elias8, Nipam H. -
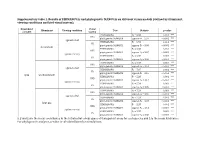
Supplementary Table 1. Results of Permanovas and Phylogenetic Manovas on Different Vision Models (Defined by Illuminant, Viewing Conditions and Bird Visual System)
Supplementary table 1. Results of PERMANOVAs and phylogenetic MANOVAs on different vision models (defined by illuminant, viewing conditions and bird visual system). Dependent Visual Illuminant Viewing condition Test Statistic p-value variable system PERMANOVA F9 = 6.88 0.001 *** UVS phylogenetic MANOVA approx-F9 = 2.97 < 0.001 *** against a leaf PERMANOVA F9 = 6.93 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.05 < 0.001 *** forest shade PERMANOVA F9 = 5.38 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** against the sky PERMANOVA F9 = 5.38 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.36 < 0.001 *** PERMANOVA F9 = 7.04 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.01 < 0.001 *** against a leaf PERMANOVA F9 = 7.07 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.10 < 0.001 *** xyzL woodland shade PERMANOVA F9 = 5.33 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.12 < 0.001 *** against the sky PERMANOVA F9 = 5.34 0.002 ** VS phylogenetic MANOVA approx-F9 = 3.39 < 0.001 *** PERMANOVA F9 = 7.24 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.00 < 0.001 *** against a leaf PERMANOVA F9 = 7.24 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** large gap PERMANOVA F9 = 5.37 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.14 < 0.001 *** against the sky PERMANOVA F9 = 5.37 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.38 < 0.001 *** x, y and z are the mean coordinates in the tetrahedral colour space of transparent areas for each species and L is the mean luminance.