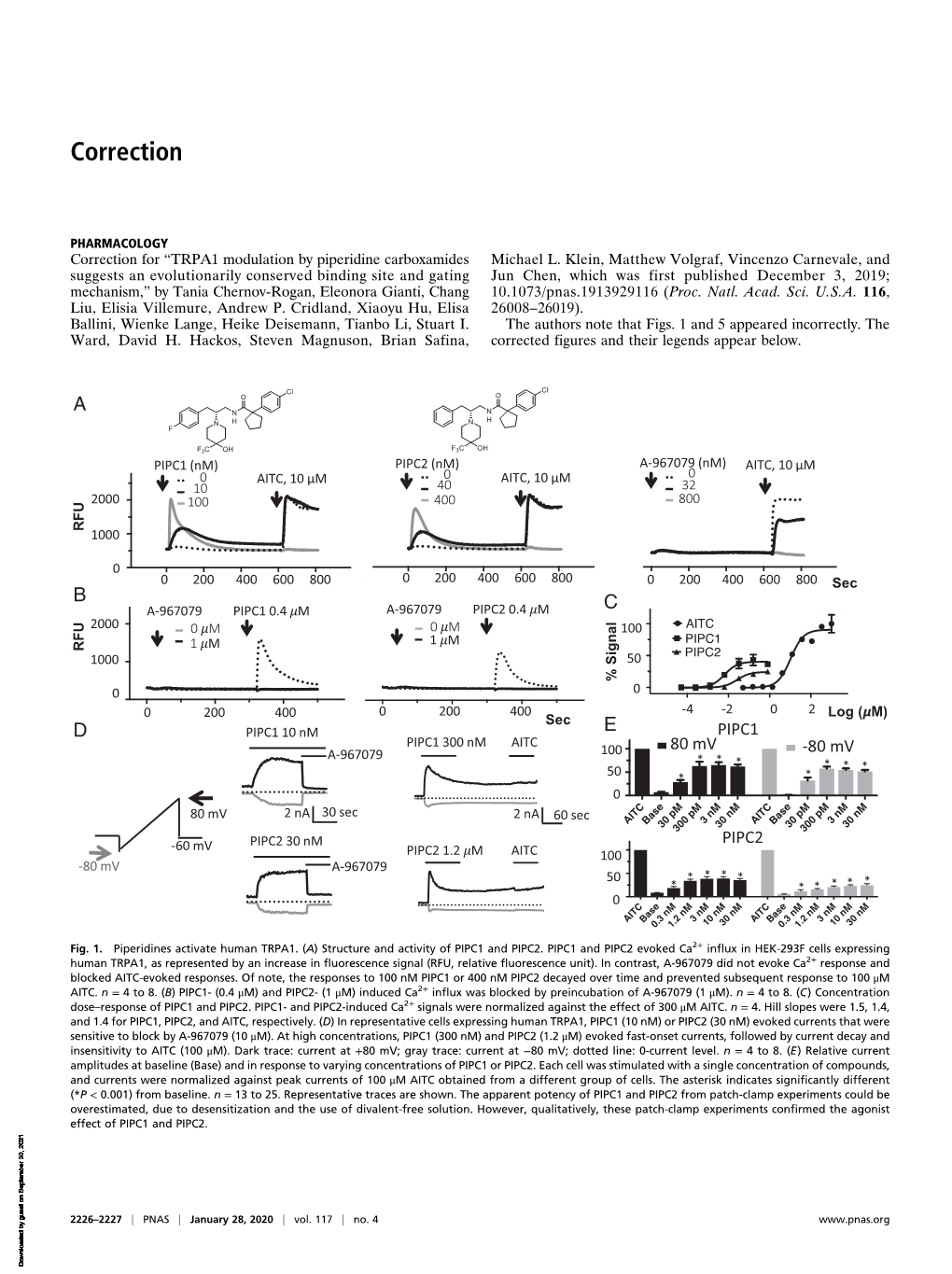
Correction for Chernov-Rogan Et Al., TRPA1 Modulation by Piperidine Carboxamides Suggests an Evolutionarily Conserved Binding Si
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Targeting the Endocannabinoid System to Reduce Nociception
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2011 Targeting the endocannabinoid system to reduce nociception Lamont Booker Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Medical Pharmacology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/2419 This Dissertation is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. Targeting the Endocannabinoid System to Reduce Nociception A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy at Virginia Commonwealth University. By Lamont Booker Bachelor’s of Science, Fayetteville State University 2003 Master’s of Toxicology, North Carolina State University 2005 Director: Dr. Aron H. Lichtman, Professor, Pharmacology & Toxicology Virginia Commonwealth University Richmond, Virginia April 2011 Acknowledgements The author wishes to thank several people. I like to thank my advisor Dr. Aron Lichtman for taking a chance and allowing me to work under his guidance. He has been a great influence not only with project and research direction, but as an excellent example of what a mentor should be (always willing to listen, understanding the needs of each student/technician, and willing to provide a hand when available). Additionally, I like to thank all of my committee members (Drs. Galya Abdrakmanova, Francine Cabral, Sandra Welch, Mike Grotewiel) for your patience and willingness to participate as a member. Our term together has truly been memorable! I owe a special thanks to Sheryol Cox, and Dr. -

Transient Receptor Potential Channel Promiscuity Frustrates Constellation
the sole sensor responsible for noxious cold responses in M+A+ LETTER neurons (1). However, TRPA1 is also activated by cooling and underlies at least part of the noxious cold responsiveness of Transient receptor potential channel AITC-sensitive neurons (3). Third, the authors used nicardipine 2+ promiscuity frustrates to selectively inhibit CaV1-type voltage-gated Ca channels (1). However, several dihydropyridines, including nicardipine, also constellation pharmacology act as TRPA1 agonists (4). These considerations led us to propose an alternative Sensory neurons from the trigeminal and dorsal root ganglia molecular interpretation of the difference between M+A− (DRG) have nerve endings in the skin and mucosa, where they and M+A+ neurons, which is in much better agreement with detect environmental stimuli and convey this information to published work. In accord with the authors, we conclude that the central nervous system. Several members of the transient M+A− neurons express TRPM8 but lack expression of receptor potential (TRP) superfamily of ion channels act as TRPA1. In contrast to the authors, we propose that M+A+ prime molecular sensors for thermal and chemical stimuli in neurons express TRPA1 as the prime cold and menthol these sensory neurons. However, it is incompletely understood sensor (2, 3). This interpretation is consistent with published how TRP channel expression and modulation affect the stimulus observations that menthol responses in M+A− but not in sensitivities of distinct neuronal subtypes. M+A+ neurons are inhibited by TRPM8 antagonists (5) In a recent article, Teichert et al. (1) described a “constellation and that TRPA1-mediated responses to cold in neurons are pharmacology approach” to identify and characterize subtypes characterized by a higher (colder) threshold (3). -

Chapter Four – TRPA1 Channels: Chemical and Temperature Sensitivity
CHAPTER FOUR TRPA1 Channels: Chemical and Temperature Sensitivity Willem J. Laursen1,2, Sviatoslav N. Bagriantsev1,* and Elena O. Gracheva1,2,* 1Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Haven, CT, USA 2Program in Cellular Neuroscience, Neurodegeneration and Repair, Yale University School of Medicine, New Haven, CT, USA *Corresponding author: E-mail: [email protected], [email protected] Contents 1. Introduction 90 2. Activation and Regulation of TRPA1 by Chemical Compounds 91 2.1 Chemical activation of TRPA1 by covalent modification 91 2.2 Noncovalent activation of TRPA1 97 2.3 Receptor-operated activation of TRPA1 99 3. Temperature Sensitivity of TRPA1 101 3.1 TRPA1 in mammals 101 3.2 TRPA1 in insects and worms 103 3.3 TRPA1 in fish, birds, reptiles, and amphibians 103 3.4 TRPA1: Molecular mechanism of temperature sensitivity 104 Acknowledgments 107 References 107 Abstract Transient receptor potential ankyrin 1 (TRPA1) is a polymodal excitatory ion channel found in sensory neurons of different organisms, ranging from worms to humans. Since its discovery as an uncharacterized transmembrane protein in human fibroblasts, TRPA1 has become one of the most intensively studied ion channels. Its function has been linked to regulation of heat and cold perception, mechanosensitivity, hearing, inflam- mation, pain, circadian rhythms, chemoreception, and other processes. Some of these proposed functions remain controversial, while others have gathered considerable experimental support. A truly polymodal ion channel, TRPA1 is activated by various stimuli, including electrophilic chemicals, oxygen, temperature, and mechanical force, yet the molecular mechanism of TRPA1 gating remains obscure. In this review, we discuss recent advances in the understanding of TRPA1 physiology, pharmacology, and molecular function. -

TRPM8 Channels and Dry Eye
UC Berkeley UC Berkeley Previously Published Works Title TRPM8 Channels and Dry Eye. Permalink https://escholarship.org/uc/item/2gz2d8s3 Journal Pharmaceuticals (Basel, Switzerland), 11(4) ISSN 1424-8247 Authors Yang, Jee Myung Wei, Edward T Kim, Seong Jin et al. Publication Date 2018-11-15 DOI 10.3390/ph11040125 Peer reviewed eScholarship.org Powered by the California Digital Library University of California pharmaceuticals Review TRPM8 Channels and Dry Eye Jee Myung Yang 1,2 , Edward T. Wei 3, Seong Jin Kim 4 and Kyung Chul Yoon 1,* 1 Department of Ophthalmology, Chonnam National University Medical School and Hospital, Gwangju 61469, Korea; [email protected] 2 Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon 34141, Korea 3 School of Public Health, University of California, Berkeley, CA 94720, USA; [email protected] 4 Department of Dermatology, Chonnam National University Medical School and Hospital, Gwangju 61469, Korea; [email protected] * Correspondence: [email protected] Received: 17 September 2018; Accepted: 12 November 2018; Published: 15 November 2018 Abstract: Transient receptor potential (TRP) channels transduce signals of chemical irritation and temperature change from the ocular surface to the brain. Dry eye disease (DED) is a multifactorial disorder wherein the eyes react to trivial stimuli with abnormal sensations, such as dryness, blurring, presence of foreign body, discomfort, irritation, and pain. There is increasing evidence of TRP channel dysfunction (i.e., TRPV1 and TRPM8) in DED pathophysiology. Here, we review some of this literature and discuss one strategy on how to manage DED using a TRPM8 agonist. -

Investigational Drugs in Early Phase Clinical Trials Targeting Thermotransient Receptor Potential (Thermotrp) Channels
Expert Opinion on Investigational Drugs ISSN: (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/ieid20 Investigational drugs in early phase clinical trials targeting thermotransient receptor potential (thermoTRP) channels Asia Fernández-Carvajal , Rosario González-Muñiz , Gregorio Fernández- Ballester & Antonio Ferrer-Montiel To cite this article: Asia Fernández-Carvajal , Rosario González-Muñiz , Gregorio Fernández- Ballester & Antonio Ferrer-Montiel (2020): Investigational drugs in early phase clinical trials targeting thermotransient receptor potential (thermoTRP) channels, Expert Opinion on Investigational Drugs, DOI: 10.1080/13543784.2020.1825680 To link to this article: https://doi.org/10.1080/13543784.2020.1825680 Published online: 29 Sep 2020. Submit your article to this journal Article views: 31 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=ieid20 EXPERT OPINION ON INVESTIGATIONAL DRUGS https://doi.org/10.1080/13543784.2020.1825680 REVIEW Investigational drugs in early phase clinical trials targeting thermotransient receptor potential (thermoTRP) channels Asia Fernández-Carvajala, Rosario González-Muñizb, Gregorio Fernández-Ballestera and Antonio Ferrer-Montiela aInstituto De Investigación, Desarrollo E Innovación En Biotecnología Sanitaria De Elche (Idibe), Universitas Miguel Hernández, Alicante, Spain; bInstituto De Química Médica, CSIC, Madrid, Spain ABSTRACT ARTICLE HISTORY Introduction: Thermo transient receptor potential (thermoTRP) channels are some of the most inten Received 15 June 2020 sely pursued therapeutic targets of the past decade. They are considered promising targets of numer Accepted 15 September ous diseases including chronic pain and cancer. Modulators of these proteins, in particular TRPV1-4, 2020 TRPM8 and TRPA1, have reached clinical development, but none has been approved for clinical practice KEYWORDS yet. -

New Natural Agonists of the Transient Receptor Potential Ankyrin 1 (TRPA1
www.nature.com/scientificreports OPEN New natural agonists of the transient receptor potential Ankyrin 1 (TRPA1) channel Coline Legrand, Jenny Meylan Merlini, Carole de Senarclens‑Bezençon & Stéphanie Michlig* The transient receptor potential (TRP) channels family are cationic channels involved in various physiological processes as pain, infammation, metabolism, swallowing function, gut motility, thermoregulation or adipogenesis. In the oral cavity, TRP channels are involved in chemesthesis, the sensory chemical transduction of spicy ingredients. Among them, TRPA1 is activated by natural molecules producing pungent, tingling or irritating sensations during their consumption. TRPA1 can be activated by diferent chemicals found in plants or spices such as the electrophiles isothiocyanates, thiosulfnates or unsaturated aldehydes. TRPA1 has been as well associated to various physiological mechanisms like gut motility, infammation or pain. Cinnamaldehyde, its well known potent agonist from cinnamon, is reported to impact metabolism and exert anti-obesity and anti-hyperglycemic efects. Recently, a structurally similar molecule to cinnamaldehyde, cuminaldehyde was shown to possess anti-obesity and anti-hyperglycemic efect as well. We hypothesized that both cinnamaldehyde and cuminaldehyde might exert this metabolic efects through TRPA1 activation and evaluated the impact of cuminaldehyde on TRPA1. The results presented here show that cuminaldehyde activates TRPA1 as well. Additionally, a new natural agonist of TRPA1, tiglic aldehyde, was identifed -

Snapshot: Mammalian TRP Channels David E
SnapShot: Mammalian TRP Channels David E. Clapham HHMI, Children’s Hospital, Department of Neurobiology, Harvard Medical School, Boston, MA 02115, USA TRP Activators Inhibitors Putative Interacting Proteins Proposed Functions Activation potentiated by PLC pathways Gd, La TRPC4, TRPC5, calmodulin, TRPC3, Homodimer is a purported stretch-sensitive ion channel; form C1 TRPP1, IP3Rs, caveolin-1, PMCA heteromeric ion channels with TRPC4 or TRPC5 in neurons -/- Pheromone receptor mechanism? Calmodulin, IP3R3, Enkurin, TRPC6 TRPC2 mice respond abnormally to urine-based olfactory C2 cues; pheromone sensing 2+ Diacylglycerol, [Ca ]I, activation potentiated BTP2, flufenamate, Gd, La TRPC1, calmodulin, PLCβ, PLCγ, IP3R, Potential role in vasoregulation and airway regulation C3 by PLC pathways RyR, SERCA, caveolin-1, αSNAP, NCX1 La (100 µM), calmidazolium, activation [Ca2+] , 2-APB, niflumic acid, TRPC1, TRPC5, calmodulin, PLCβ, TRPC4-/- mice have abnormalities in endothelial-based vessel C4 i potentiated by PLC pathways DIDS, La (mM) NHERF1, IP3R permeability La (100 µM), activation potentiated by PLC 2-APB, flufenamate, La (mM) TRPC1, TRPC4, calmodulin, PLCβ, No phenotype yet reported in TRPC5-/- mice; potentially C5 pathways, nitric oxide NHERF1/2, ZO-1, IP3R regulates growth cones and neurite extension 2+ Diacylglycerol, [Ca ]I, 20-HETE, activation 2-APB, amiloride, Cd, La, Gd Calmodulin, TRPC3, TRPC7, FKBP12 Missense mutation in human focal segmental glomerulo- C6 potentiated by PLC pathways sclerosis (FSGS); abnormal vasoregulation in TRPC6-/- -

Macromolecular Assembly of Polycystin-2 Intracytosolic C-Terminal Domain
Macromolecular assembly of polycystin-2 intracytosolic C-terminal domain Frederico M. Ferreiraa,b,1, Leandro C. Oliveirac, Gregory G. Germinod, José N. Onuchicc,1, and Luiz F. Onuchica,1 aDivision of Nephrology, University of São Paulo School of Medicine, 01246-903, São Paulo, Brazil; bLaboratory of Immunology, Heart Institute, University of São Paulo School of Medicine, 05403-900, São Paulo, Brazil; cCenter for Theoretical Biological Physics, University of California at San Diego, La Jolla, CA 92093; dNational Institute of Diabetes, Digestive, and Kidney Diseases, Bethesda, MD 20892-2560 Contributed by José N. Onuchic, April 28, 2011 (sent for review March 20, 2011) Mutations in PKD2 are responsible for approximately 15% of the In spite of the aforementioned information and insights, the autosomal dominant polycystic kidney disease cases. This gene macromolecular assembly of PC2t homooligomer continued to encodes polycystin-2, a calcium-permeable cation channel whose be an open question. In the current work, we present the most C-terminal intracytosolic tail (PC2t) plays an important role in its comprehensive set of analyses yet performed and that show interaction with a number of different proteins. In the present PC2t forms a homotetrameric oligomer. We have proposed a study, we have comprehensively evaluated the macromolecular PC2 C-terminal domain delimitation and submitted it to a range assembly of PC2t homooligomer using a series of biophysical of biochemical and biophysical evaluations, including chemical and biochemical analyses. Our studies, based on a new delimitation cross-linking, dynamic light scattering (DLS), circular dichroism of PC2t, have revealed that it is capable of assembling as a homo- (CD) and small angle X-ray scattering (SAXS) analyses. -

(TRPV2, TRPV3, TRPV4, TRPV5, and TRPV6) Gene and Protein Expression in Patients with Ulcerative Colitis
Hindawi Journal of Immunology Research Volume 2020, Article ID 2906845, 11 pages https://doi.org/10.1155/2020/2906845 Research Article TRPV Subfamily (TRPV2, TRPV3, TRPV4, TRPV5, and TRPV6) Gene and Protein Expression in Patients with Ulcerative Colitis Joel J. Toledo Mauriño,1,2 Gabriela Fonseca-Camarillo ,1 Janette Furuzawa-Carballeda ,3 Rafael Barreto-Zuñiga,4 Braulio Martínez Benítez ,5 Julio Granados ,6 and Jesus K. Yamamoto-Furusho 1 1Inflammatory Bowel Disease Clinic. Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico 2MD/PhD Program (PECEM), Facultad de Medicina, Universidad Nacional Autónoma de México, Av. Ciudad Universitaria 3000, C.P. 04360 Coyoacán, México City, Mexico 3Department of Immunology and Rheumatology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico 4Department of Endoscopy, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico 5Department of Pathology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico 6Department of Transplantation, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico Correspondence should be addressed to Jesus K. Yamamoto-Furusho; [email protected] Received 25 October 2019; Revised 4 March 2020; Accepted 11 April 2020; Published 8 May 2020 Academic Editor: Francesca Santilli Copyright © 2020 Joel J. Toledo Mauriño et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Introduction. TRPVs are a group of receptors with a channel activity predominantly permeable to Ca2+. This subfamily is involved in the development of gastrointestinal diseases such as ulcerative colitis (UC). -

Ion Channels
UC Davis UC Davis Previously Published Works Title THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels. Permalink https://escholarship.org/uc/item/1442g5hg Journal British journal of pharmacology, 176 Suppl 1(S1) ISSN 0007-1188 Authors Alexander, Stephen PH Mathie, Alistair Peters, John A et al. Publication Date 2019-12-01 DOI 10.1111/bph.14749 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology (2019) 176, S142–S228 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels Stephen PH Alexander1 , Alistair Mathie2 ,JohnAPeters3 , Emma L Veale2 , Jörg Striessnig4 , Eamonn Kelly5, Jane F Armstrong6 , Elena Faccenda6 ,SimonDHarding6 ,AdamJPawson6 , Joanna L Sharman6 , Christopher Southan6 , Jamie A Davies6 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK 2Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building, Central Avenue, Chatham Maritime, Chatham, Kent, ME4 4TB, UK 3Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK 4Pharmacology and Toxicology, Institute of Pharmacy, University of Innsbruck, A-6020 Innsbruck, Austria 5School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, BS8 1TD, UK 6Centre for Discovery Brain Science, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2019/20 is the fourth in this series of biennial publications. The Concise Guide provides concise overviews of the key properties of nearly 1800 human drug targets with an emphasis on selective pharmacology (where available), plus links to the open access knowledgebase source of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. -

Microarray Analysis Reveals the Inhibition of Intestinal Expression Of
www.nature.com/scientificreports OPEN Microarray analysis reveals the inhibition of intestinal expression of nutrient transporters in piglets infected with porcine epidemic diarrhea virus Junmei Zhang1,3, Di Zhao1,3, Dan Yi1,3, Mengjun Wu1, Hongbo Chen1, Tao Wu1, Jia Zhou1, Peng Li1, Yongqing Hou1* & Guoyao Wu2 Porcine epidemic diarrhea virus (PEDV) infection can induce intestinal dysfunction, resulting in severe diarrhea and even death, but the mode of action underlying these viral efects remains unclear. This study determined the efects of PEDV infection on intestinal absorption and the expression of genes for nutrient transporters via biochemical tests and microarray analysis. Sixteen 7-day-old healthy piglets fed a milk replacer were randomly allocated to one of two groups. After 5-day adaption, piglets (n = 8/ group) were orally administrated with either sterile saline or PEDV (the strain from Yunnan province) 4.5 at 10 TCID50 (50% tissue culture infectious dose) per pig. All pigs were orally infused D-xylose (0.1 g/ kg BW) on day 5 post PEDV or saline administration. One hour later, jugular vein blood samples as well as intestinal samples were collected for further analysis. In comparison with the control group, PEDV infection increased diarrhea incidence, blood diamine oxidase activity, and iFABP level, while reducing growth and plasma D-xylose concentration in piglets. Moreover, PEDV infection altered plasma and jejunal amino acid profles, and decreased the expression of aquaporins and amino acid transporters (L-type amino acid -

The Ca2+-Permeable Cation Transient Receptor Potential
1521-0111/92/3/193–200$25.00 https://doi.org/10.1124/mol.116.107946 MOLECULAR PHARMACOLOGY Mol Pharmacol 92:193–200, September 2017 Copyright ª 2017 by The American Society for Pharmacology and Experimental Therapeutics MINIREVIEW—MOLECULAR PHARMACOLOGY IN CHINA The Ca21-Permeable Cation Transient Receptor Potential TRPV3 Channel: An Emerging Pivotal Target for Itch and Skin Diseases Gongxin Wang and KeWei Wang Department of Pharmacology, Qingdao University School of Pharmacy and Institute of Innovative Drugs, Qingdao University, Downloaded from Qingdao, Shandong Province, China Received December 20, 2016; accepted March 31, 2017 ABSTRACT Temperature-sensitive transient receptor potential (TRP) chan- syndrome, which is characterized by severe itching and molpharm.aspetjournals.org nels such as TRPA1 and TRPV1 have been identified as down- palmoplantar and periorificial keratoderma, unveils its crucial stream ion channel targets in the transduction of itch. As a member role in chronic itch and skin diseases. In this review, we will of the temperature-sensitive TRP family, the Ca21-permeable focus on recent progress made in the understanding of nonselective cation channel TRPV3 is expressed abundantly TRPV3 that emerges as an attractive target for developing in skin keratinocytes. Recent identification of gain-of-function effective antipruritic therapy for chronic itch or skin-related mutations of human TRPV3 from patients with Olmsted diseases. attractive target for developing antipruritic therapy in chronic Introduction at ASPET Journals on September 28, 2021 itch or skin-related diseases. Itch (also known as pruritus) is an unpleasant sensation of The superfamily of TRP channels is composed of 28 mam- the skin, provoking the desire or reflex to scratch.