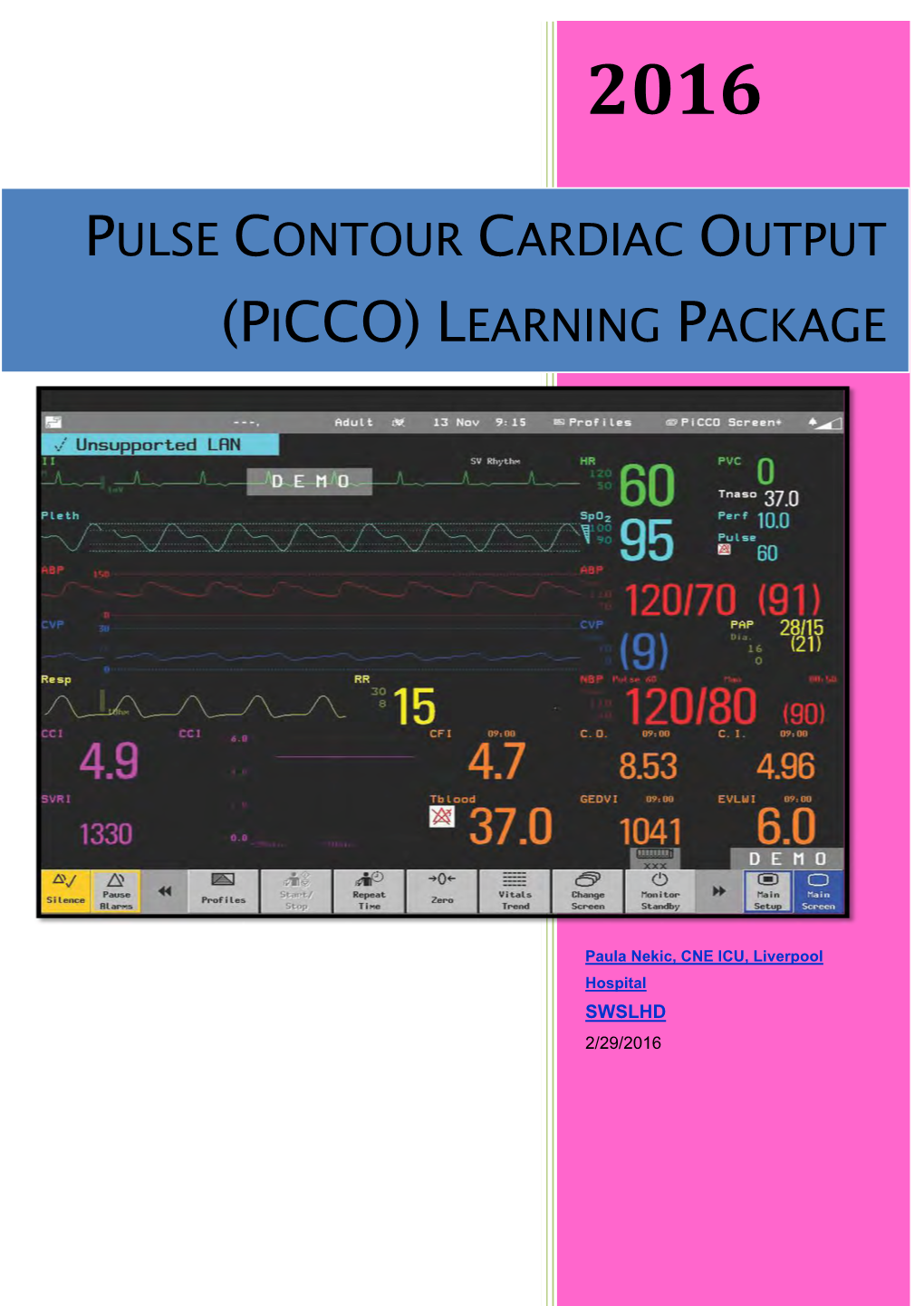
Pulse Contour Cardiac Output (Picco) Learning Package
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Angiotensin II Protocol
Angiotensin II (Giapreza ™) Protocol Background Sepsis and septic shock are medical emergencies that affect millions of people each year and killing as many as 1 in 4.1 The cornerstones of therapy are fluid resuscitation, early appropriate antibiotics, source control if needed and vasopressors. A small portion of patients fail to respond to these therapies and develop refractory shock. The definition of refractory septic shock varies in the literature but is generally considered to be hypotension, with end-organ dysfunction, requiring high-dose vasopressor support.2 The associated mortality of refractory septic shock is up to 60% and as high as 80-90% in patients requiring more than 1 mcg/kg/min of norepinephrine.2,3 Patients who develop refractory septic shock comprise a very small portion of the population in large randomized controlled trials therefore limited data is available regarding outcomes and management. Indications: Angiotensin II (Ang II) is a vasoconstrictor used to increase blood pressure in adults with septic or other distributive shock. Administration: Starting dose of 5 (nanograms) ng/kg/min intravenously via central line only. Titration: Every 5 minutes by increments of 5 ng/kg/min as needed. Maximum dose should not exceed 80 ng/kg/min (During the first 3 hours of administration); after the first 3 hours the maintenance (maximum) dose is 40 ng/kg/min. Monitoring: Critical care setting only with telemetry, arterial blood pressure, and continuous SpO2 monitoring. DVT Prophylaxis should be started (unless contraindicated) -

Pleth Variability Index: a Dynamic Measurement to Help Assess to Help Measurement a Dynamic Index: Variability Pleth Responsiveness and Fluid Physiology
Pleth Variability Index: A Dynamic Measurement to Help Assess TECHNICAL BULLETIN TECHNICAL Physiology and Fluid Responsiveness SUMMARY PVI®, an index available with Masimo SET® pulse oximetry, is the first and only noninvasive, continuous, easy-to-use method to help clinicians manage fluid responsiveness in sedated patients under positive pressure ventilation. Other clinical uses have also been developed for PVI, including helping clinicians to assess the effects of positive end expiratory pressure on cardiac index and to identify patients at risk for hypotension during anesthesia induction. PVI, along with the other innovative noninvasive monitoring technologies available with the Masimo rainbow SET® platform (SpHb®, SpCO®, SpMet®, RRa™), has helped clinicians to realize improved patient outcomes while lowering the cost of care. INTRODUCTION Many pulse oximetry technologies display a processed and filtered representation of the photoplethysmosgraphic waveform. Each manufacturer uses unique proprietary algorithms to calculate the waveform displayed on the pulse oximeter. Masimo has extracted and processed information from the waveform to create two physiologic indices, perfusion index (PI) and pleth variability index (PVI). PI is calculated by indexing the infrared (IR) pulsatile signal against the nonpulsitile signal and expressing this number as a percentage. Masimo SET® PI has been shown to be useful to gauge the severity of illness in newborns1, 2 to assess the effectiveness of epidural blocks3, 4 to indicate successful interscalene nerve block placement in awake patients5 and to quantify peripheral perfusion for diagnosis of congenital heart disease in newborns.6 PVI is the first and only commercially available measurement that automatically and continuously calculates the respiratory variations in the photoplethysmosgraphic waveform. -

Pulse Wave Analysis to Estimate Cardiac Output
CLINICAL FOCUS REVIEW Jerrold H. Levy, M.D., F.A.H.A., F.C.C.M., Editor Pulse Wave Analysis to Estimate Cardiac Output Karim Kouz, M.D., Thomas W. L. Scheeren, M.D., Daniel de Backer, M.D., Bernd Saugel, M.D. ardiac output (CO)–guided therapy is a promising reference CO value measured using an indicator dilution Capproach to hemodynamic management in high-risk method (transpulmonary thermodilution or lithium dilu- patients having major surgery1 and in critically ill patients tion).5,9 CO measurement using indicator dilution methods with circulatory shock.2 Pulmonary artery thermodilu- requires a (central) venous catheter for indicator injection tion remains the clinical reference method for CO mea- upstream in the circulation and a dedicated arterial catheter Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/134/1/119/513741/20210100.0-00023.pdf by guest on 29 September 2021 surement,3 but the use of the pulmonary artery catheter and measurement system to detect downstream indicator decreased over the past two decades.4 Today, various CO temperature or concentration changes.5,9–11 monitoring methods with different degrees of invasiveness The VolumeView system (Edwards Lifesciences, are available, including pulse wave analysis.5 Pulse wave USA) and the PiCCO system (Pulsion Medical Systems, analysis is the mathematical analysis of the arterial blood Germany) calibrate pulse wave analysis–derived CO to pressure waveform and enables CO to be estimated con- transpulmonary thermodilution–derived CO. To measure tinuously and in real time.6 In this article, we review pulse CO using transpulmonary thermodilution, a bolus of cold wave analysis methods for CO estimation, including their crystalloid solution is injected in the central venous circu- underlying measurement principles and their clinical appli- lation.10 The cold indicator bolus injection causes changes cation in perioperative and intensive care medicine. -

Chapter 9 Monitoring of the Heart and Vascular System
Chapter 9 Monitoring of the Heart and Vascular System David L. Reich, MD • Alexander J. Mittnacht, MD • Martin J. London, MD • Joel A. Kaplan, MD Hemodynamic Monitoring Cardiac Output Monitoring Arterial Pressure Monitoring Indicator Dilution Arterial Cannulation Sites Analysis and Interpretation Indications of Hemodynamic Data Insertion Techniques Systemic and Pulmonary Vascular Resistances Central Venous Pressure Monitoring Frank-Starling Relationships Indications Monitoring Coronary Perfusion Complications Electrocardiography Pulmonary Arterial Pressure Monitoring Lead Systems Placement of the Pulmonary Artery Catheter Detection of Myocardial Ischemia Indications Intraoperative Lead Systems Complications Arrhythmia and Pacemaker Detection Pacing Catheters Mixed Venous Oxygen Saturation Catheters Summary References HEMODYNAMIC MONITORING For patients with severe cardiovascular disease and those undergoing surgery associ- ated with rapid hemodynamic changes, adequate hemodynamic monitoring should be available at all times. With the ability to measure and record almost all vital physi- ologic parameters, the development of acute hemodynamic changes may be observed and corrective action may be taken in an attempt to correct adverse hemodynamics and improve outcome. Although outcome changes are difficult to prove, it is a rea- sonable assumption that appropriate hemodynamic monitoring should reduce the incidence of major cardiovascular complications. This is based on the presumption that the data obtained from these monitors are interpreted correctly and that thera- peutic decisions are implemented in a timely fashion. Many devices are available to monitor the cardiovascular system. These devices range from those that are completely noninvasive, such as the blood pressure (BP) cuff and ECG, to those that are extremely invasive, such as the pulmonary artery (PA) catheter. To make the best use of invasive monitors, the potential benefits to be gained from the information must outweigh the potential complications. -

Inorganic Nitrate As a Treatment for Acute Heart Failure
Falls et al. J Transl Med (2017) 15:172 DOI 10.1186/s12967-017-1271-z Journal of Translational Medicine PROTOCOL Open Access Inorganic nitrate as a treatment for acute heart failure: a protocol for a single center, randomized, double‑blind, placebo‑controlled pilot and feasibility study Roman Falls1,2, Michael Seman1,2, Sabine Braat2,4, Joshua Sortino1, Jason D. Allen1,3 and Christopher J. Neil1,2,3,5* Abstract Background: Acute heart failure (AHF) is a frequent reason for hospitalization worldwide and efective treatment options are limited. It is known that AHF is a condition characterized by impaired vasorelaxation, together with reduced nitric oxide (NO) bioavailability, an endogenous vasodilatory compound. Supplementation of inorganic sodium nitrate (NaNO3) is an indirect dietary source of NO, through bioconversion. It is proposed that oral sodium nitrate will favorably afect levels of circulating NO precursors (nitrate and nitrite) in AHF patients, resulting in reduced systemic vascular resistance, without signifcant hypotension. Methods and outcomes: We propose a single center, randomized, double-blind, placebo-controlled pilot trial, evaluating the feasibility of sodium nitrate as a treatment for AHF. The primary hypothesis that sodium nitrate treat- ment will result in increased systemic levels of nitric oxide pre-cursors (nitrate and nitrite) in plasma, in parallel with improved vasorelaxation, as assessed by non-invasively derived systemic vascular resistance index. Additional sur- rogate measures relevant to the known pathophysiology of AHF will be obtained in order to assess clinical efect on dyspnea and renal function. Discussion: The results of this study will provide evidence of the feasibility of this novel approach and will be of inter- est to the heart failure community. -

Heartlogic Clinical Compendium Updated As of February 2020 TABLE of CONTENTS
HeartLogic Clinical Compendium Updated as of February 2020 TABLE OF CONTENTS Introduction.............................................................................................. 2 HeartLogic Heart Failure Diagnostic ................................................... 3 Heart Sounds ......................................................................................... 11 Respiration ............................................................................................. 19 Thoracic Impedance ............................................................................ 23 Activity Level.......................................................................................... 25 Night Heart Rate ................................................................................... 26 Sleep Incline .......................................................................................... 27 Weight ..................................................................................................... 29 RV and LV Pacing .................................................................................. 30 Arrhythmia Burden ............................................................................... 33 Heart Failure Assessment ................................................................... 37 THIS DOCUMENT IS A COMPILATION OF RELEVANT CLINICAL PUBLICATION REFERENCES THAT FORM THE CLINICAL FOUNDATION OF THE HEARTLOGIC HEART FAILURE DIAGNOSTIC. PUBLICATIONS ARE LISTED BY SENSOR TREND/TOPIC AREA AND THEN BY RELEVANCE ON FEASIBILITY, -

The Relation Between Cardiac Output and Body Size*
Br Heart J: first published as 10.1136/hrt.25.4.425 on 1 July 1963. Downloaded from Brit. Heart J., 1963, 25, 425. THE RELATION BETWEEN CARDIAC OUTPUT AND BODY SIZE* BY W. JEGIER, PAUL SEKELJ, P. A. M. AULD, R. SIMPSON, AND M. McGREGOR From the Joint Cardio-Respiratory Service of the Montreal Children's Hospital and the Royal Victoria Hospital, The Department of Anesthesia, The Montreal Children's Hospital, and the Department ofPhysiology, McGill University, Montreal, Quebec, Canada Received December 10, 1962 A prerequisite in the study of abnormal body function is the ability to establish the limits of normal. In the case of parameters such as cardiac output that vary with the size of the subject, it has become an accepted practice to standardize values in relation to the body surface area. Thus the cardiac index describes the cardiac output per square metre of body surface area, and the stroke index describes the volume of blood per heart beat per square metre of body surface area. The validity of these expressions depends on the premise that there is a constant or straight line relation between body surface area on the one hand, and cardiac output and stroke volume on the other, over the whole range of body size to be studied, and that the relation can be described by a simple regression equation cutting the intercept at zero. Only where this is so is it meaningful to refer to "the normal" cardiac index or stroke index. Though this premise is backed by observations in the case of adult or adolescent subjects, it was until recently entirely unsupported in the case of children and is still unsupported by any data for infants. -

Anemia on Cardiovascular Hemodynamics, Therapeutic Strategy and Clinical Outcomes in Patients with Heart Failure and Hemodynamic Congestion
1670 TANIMURA M et al. Circ J 2017; 81: 1670 – 1677 ORIGINAL ARTICLE doi: 10.1253/circj.CJ-17-0171 Heart Failure Effect of Anemia on Cardiovascular Hemodynamics, Therapeutic Strategy and Clinical Outcomes in Patients With Heart Failure and Hemodynamic Congestion Muneyoshi Tanimura, MD; Kaoru Dohi, MD, PhD; Naoki Fujimoto, MD, PhD; Keishi Moriwaki, MD; Taku Omori, MD; Yuichi Sato, MD, PhD; Emiyo Sugiura, MD, PhD; Naoto Kumagai, MD, PhD; Shiro Nakamori, MD, PhD; Tairo Kurita, MD, PhD; Eitaro Fujii, MD, PhD; Norikazu Yamada, MD, PhD; Masaaki Ito, MD, PhD Background: We investigated the effect of anemia on cardiovascular hemodynamics, therapeutic strategies and clinical outcomes in heart failure (HF) patients. Methods and Results: We divided 198 consecutive HF patients who underwent right heart catheterization before in-hospital HF treatment into 2 groups according to the presence or absence of hemodynamic congestion (HC: mean pulmonary capillary wedge pressure ≥15 mmHg and/or mean right atrial pressure ≥10 mmHg). The hemoglobin level correlated with the cardiac index (CI) and systemic vascular resistance index (SVRI) (r=−0.34 and 0.42, P<0.05, respectively), and was the strongest contributor of SVRI only in the HC group. Anemic patients more frequently required intravenous inotropic support despite having higher CI and lower SVRI than non-anemic patients in the HC group. The novel hemodynamic subsets based on mean right atrial pressure and estimated left ventricular stroke work index but not Forrester subsets appropriately predicted the need for intravenous inotropic support. The prob- ability of hospitalization for worsening HF during 2-year follow-up period was significantly higher in anemic patients than in non- anemic patients in the HC group. -

Diagnosis and Management of Acute Heart Failure
860 Consensus Report Diagnosis and management of acute heart failure Dilek Ural, Yüksel Çavuşoğlu1, Mehmet Eren2, Kurtuluş Karaüzüm, Ahmet Temizhan3, Mehmet Birhan Yılmaz4, Mehdi Zoghi5, Kumudha Ramassubu6, Biykem Bozkurt6 Department of Cardiology, Medical Faculty of Kocaeli University; Kocaeli-Turkey; 1Department of Cardiology, Medical Faculty of Eskişehir Osmangazi University; Eskişehir-Turkey; 2Department of Cardiology, Siyami Ersek Hospital; İstanbul-Turkey; 3Department of Cardiology, Turkey Yüksek İhtisas Hospital; Ankara-Turkey; 4Department of Cardiology, Medical Faculty of Cumhuriyet University; Sivas-Turkey; 5Department of Cardiology, Medical Faculty of Ege University; İzmir-Turkey 6Department of Cardiology, Baylor College of Medicine and University of Texas Medical School; Texas-USA ABSTRACT Acute heart failure (AHF) is a life threatening clinical syndrome with a progressively increasing incidence in general population. Turkey is a country with a high cardiovascular mortality and recent national statistics show that the population structure has turned to an 'aged' population. As a consequence, AHF has become one of the main reasons of admission to cardiology clinics. This consensus report summarizes clinical and prognostic classification of AHF, its worldwide and national epidemiology, diagnostic work-up, principles of approach in emergency department, intensive care unit and ward, treatment in different clinical scenarios and approach in special conditions and how to plan hospital discharge. (Anatol J Cardiol 2015: 15; 860-89) Keywords: acute heart failure, diagnosis, management 1. Introduction of Academic Emergency Medicine (5) do also take attention of cardiologists. However, Turkish AHF patients show some epide- Acute heart failure (AHF) is defined as a life threatening clini- miological differences than European or American AHF patients cal syndrome with rapidly developing or worsening typical heart and some pharmacological (e.g. -

Cardiovascular Failure, Inotropes and Vasopressors
Cardiovascular failure, inotropes and vasopressors Introduction thetensionintheventricularwallduring nantly affected by extrinsic factors sum- Cardiovascularfailure(‘shock’)meansthat diastoleastheheartfillswithbloodresult- marizedinTable 2. tissue perfusion is inadequate to meet inginstretchingofcardiacmusclefibres. metabolicdemandsforoxygenandnutri- Stretchingthefibresincreasestheforceof Oxygen delivery ents.Ifuncorrectedthiscanleadtoirre- contractionduringthesubsequentsystole Adequate oxygen delivery is dependent versible tissue hypoxia and cell death. (Frank–Starlingmechanismoftheheart). onboththecardiacoutputandthearte- Cardiovascularfailureisacommonindica- Afterloadisthetensionintheventricu- rial oxygen content. Most oxygen trans- tionforadmissiontothecriticalcareunit. lar wall required to eject blood into the portedinthebloodisboundtohaemo- Theaimoftreatmentistosupporttissue aorta. This will vary depending on the globin.Agramoffully-saturatedhaemo- perfusionandoxygendeliverywhichcan volume of the ventricle, the thickness of globin can carry 1.34ml of oxygen. beachievedthroughtheuseofvasoactive thewall,increasedsystemicvascularresist- Oxygen will also be dissolved in the drugs(inotropesandvasopressors). ance and the presence of conditions that plasma but the amount is negligible at Inotropes increase cardiac contractility obstructoutflow(e.g.aorticstenosis). normalatmosphericpressuresandthere- and cardiac output while vasopressors Contractility is the intrinsic ability of fore disregarded. Therefore the arterial cause vasoconstriction -

Pulse Pressure As a Haemodynamic Variable in Systolic Heart Failure Petrie, Colin James
University of Groningen Pulse pressure as a haemodynamic variable in systolic heart failure Petrie, Colin James IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2016 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Petrie, C. J. (2016). Pulse pressure as a haemodynamic variable in systolic heart failure. University of Groningen. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license. More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne- amendment. Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 02-10-2021 CHAPTER 5 A low pulse pressure as a poor-mans indicator of a low cardiac index in patients with severe cardiac dysfunction. -

INDE 221 Spring 2010 Syllabus Part 2
Human Health & Disease Mondays, Tuesdays, Thursdays and Fridays 9:00 - 11:50 AM Lectures: Room M-112, Labs: Fleischmann IINNDDEE 222211 SSpprriinngg 22001100 Syllabus SSyyllllaabbuuss PPaarrtt 22 (2010) Year's Last Syllabus (2010) Year's Last Human Health & Disease Inde 221 Spring 2010 Table of Contents CARDIOVASCULAR BLOCK SYLLABUS SCHEDULE 7 SYLLABUS PREFACE 11 CARDIAC MUSCLE AND FHC 15 EXCITATION-CONTRACTION COUPLING Syllabus27 NERNST POTENTIAL AND OSMOSIS 43 EXCITABILITY AND CONDUCTION 51 CIRCULATORY VESSEL HISTOLOGY LAB 63 CARDIAC ACTION POTENTIAL 71 CONTROL OF HEART RHYTHM (2010) 85 AUTONOMIC DRUGS OVERVIEW I 97 ELECTROCARDIOGRAM (ECG) 99 LESIONS OF BLOOD VESSELS 109 THROMBOEMBOLIC DISEASE 117 CARDIAC REFLEXESYear's 123 AUTONOMIC DRUGS OVERVIEW II 141 ECG SMALL GROUPS 143 LastAUTONOMIC DRUGS: CHOLINERGICS 147 CARDIAC MUSCLE MECHANICS 149 AUTONOMIC DRUGS: ANTICHOLINERGICS 175 ARRHYTHMIAS 177 AUTONOMIC DRUGS: SYMPATHOMIMETICS I 203 VENTRICULAR PHYSIOLOGY 205 AUTONOMIC DRUGS: SYMPATHOMIMETICS II 235 STARLING CURVE AND VENOUS RETURN 237 CARDIAC OUTPUT AND CATHETERIZATION 245 AUTONOMIC DRUGS: ADRENOCEPTOR BLOCKERS 267 PHYSICS OF CIRCULATION Syllabus269 CASE DISCUSSIONS: AUTONOMIC DRUGS 289 SMOOTH MUSCLE 291 ISCHEMIC AND VALVULAR HEART DISEASE 303 RENAL CIRCULATION (2010) 323 HYPERTENSION 333 CARDIOMYOPATHY, MYOCARDITIS AND ATRIAL MYXOMA 351 ENDOTHELIUM AND CORONARY CIRCULATION 369 ANGINA PECTORIS 389 DRUGS USEDYear's IN HYPERTENSION 397 SHOCK 399 ADULT CARDIAC LAB 413 CARDIAC ANESTHESIA & BYPASS 421 LastEXERCISE PHYSIOLOGY 431 ISCHEMIC