ZOSTAVAX (Zoster Vaccine Live) Refrigerated Package Insert
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

(ACIP) General Best Guidance for Immunization
9. Special Situations Updates Major revisions to this section of the best practices guidance include the timing of intramuscular administration and the timing of clotting factor deficiency replacement. Concurrent Administration of Antimicrobial Agents and Vaccines With a few exceptions, use of an antimicrobial agent does not interfere with the effectiveness of vaccination. Antibacterial agents have no effect on inactivated, recombinant subunit, or polysaccharide vaccines or toxoids. They also have no effect on response to live, attenuated vaccines, except BCG vaccines. Antimicrobial or immunosuppressive agents may interfere with the immune response to BCG and should only be used under medical supervision (for additional information, see www.merck.com/product/usa/pi_circulars/b/bcg/bcg_pi.pdf). Antiviral drugs used for treatment or prophylaxis of influenza virus infections have no effect on the response to inactivated influenza vaccine (2). However, live, attenuated influenza vaccine should not be administered until 48 hours after cessation of therapy with antiviral influenza drugs. If feasible, to avoid possible reduction in vaccine effectiveness, antiviral medication should not be administered for 14 days after LAIV administration (2). If influenza antiviral medications are administered within 2 weeks after receipt of LAIV, the LAIV dose should be repeated 48 or more hours after the last dose of zanamavir or oseltamivir. The LAIV dose should be repeated 5 days after peramivir and 17 days after baloxavir. Alternatively, persons receiving antiviral drugs within the period 2 days before to 14 days after vaccination with LAIV may be revaccinated with another approved vaccine formulation (e.g., IIV or recombinant influenza vaccine). Antiviral drugs active against herpesviruses (e.g., acyclovir or valacyclovir) might reduce the efficacy of vaccines containing live, attenuated varicella zoster virus (i.e., Varivax and ProQuad) (3,4). -

Truvada (Emtricitabine / Tenofovir Disoproxil)
Pre-exposure Prophylaxis (2.3) HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Recommended dose in HIV-1 uninfected adults: One tablet TRUVADA safely and effectively. See full prescribing information (containing 200 mg/300 mg of emtricitabine and tenofovir for TRUVADA. disoproxil fumarate) once daily taken orally with or without food. (2.3) TRUVADA® (emtricitabine/tenofovir disoproxil fumarate) tablets, for oral use Recommended dose in renally impaired HIV-uninfected Initial U.S. Approval: 2004 individuals: Do not use TRUVADA in HIV-uninfected individuals if CrCl is below 60 mL/min. If a decrease in CrCl is observed in WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH uninfected individuals while using TRUVADA for PrEP, evaluate STEATOSIS, POST-TREATMENT ACUTE EXACERBATION OF potential causes and re-assess potential risks and benefits of HEPATITIS B, and RISK OF DRUG RESISTANCE WITH USE OF continued use. (2.4) TRUVADA FOR PrEP IN UNDIAGNOSED HIV-1 INFECTION -----------------------DOSAGE FORMS AND STRENGTHS------------------- See full prescribing information for complete boxed warning. Tablets: 200 mg/300 mg, 167 mg/250 mg, 133 mg/200 mg, and 100 Lactic acidosis and severe hepatomegaly with steatosis, mg/150 mg of emtricitabine and tenofovir disoproxil fumarate . (3) including fatal cases, have been reported with the use of nucleoside analogs, including VIREAD, a component of TRUVADA. (5.1) --------------------------------CONTRAINDICATIONS----------------------------- TRUVADA is not approved for the treatment of chronic Do not use TRUVADA for pre-exposure prophylaxis in individuals with hepatitis B virus (HBV) infection. Severe acute unknown or positive HIV-1 status. TRUVADA should be used in exacerbations of hepatitis B have been reported in patients HIV-infected patients only in combination with other antiretroviral coinfected with HIV-1 and HBV who have discontinued agents. -

Which Drugs Are Most Effective for Recurrent Herpes Labialis?
Evidence-based answers from the clinical inquiries Family Physicians Inquiries Network Eiko Tubridy, MD; Gary Kelsberg, MD Valley Family Residency Which drugs are most Program, Renton, Wash Leilani St Anna, MLIS, effective for recurrent AHIP University of Washington Health Sciences Libraries, herpes labialis? Seattle AssistanT EDITOR EvidEncE-basEd answEr Jon O. neher, MD Valley Family Residency daily oral acyclovir or vala- ing to the agent used: valacyclovir reduces Program, Renton, Wash A cyclovir may help prevent her- both healing time and duration of pain, pes simplex labialis (HSL) recurrences famciclovir reduces both in one dosage (strength of recommendation [SOR]: B, form but not another, and acyclovir reduces meta-analysis of randomized controlled only pain duration (SOR: B, single RCTs). trials [RCTs] with heterogeneous results). Several topical medications (acyclovir, No trials compare oral or topical treat- penciclovir, docosanol) modestly decrease ments for HSL outbreaks against each oth- healing time and pain duration—typically er. Oral antivirals modestly reduce healing by less than a day—and require multiple time and duration of pain, varying accord- doses per day (SOR: B, multiple RCTs). Evidence summary The authors of the meta-analysis noted A systematic review and meta-analysis of the that although 9 studies favored the use of an effectiveness of oral and topical nucleoside antiviral drug, only 4 showed statistically sig- antiviral agents to prevent recurrent HSL in nificant differences when compared with pla- immunocompetent people found 11 RCTs cebo, and none of them had a low risk of bias. with a total of 1250 patients that compared They concluded that the review supported us- an active drug against placebo.1 The medi- ing oral acyclovir and valacyclovir to prevent cations were topical 5% acyclovir, topical 1% recurrent HSL.1 penciclovir, and oral acyclovir, valacyclovir, or famciclovir in various doses. -

Reference ID: 2998411
• New onset or worsening renal impairment: Can include acute HIGHLIGHTS OF PRESCRIBING INFORMATION renal failure and Fanconi syndrome. Assess creatinine clearance These highlights do not include all the information needed to use (CrCl) before initiating treatment with COMPLERA. Monitor CrCl COMPLERA safely and effectively. See full prescribing and serum phosphorus in patients at risk. Avoid administering information for COMPLERA. COMPLERA with concurrent or recent use of nephrotoxic drugs. (5.3) COMPLERATM (emtricitabine/rilpivirine/tenofovir disoproxil • Caution should be given to prescribing COMPLERA with drugs fumarate) tablets that may reduce the exposure of rilpivirine. (5.4) Initial U.S. Approval: 2011 • Caution should be given to prescribing COMPLERA with drugs with a known risk of Torsade de Pointes. (5.4) WARNINGS: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT ACUTE • Depressive disorders: Severe depressive disorders (depressed EXACERBATION OF HEPATITIS B mood, depression, dysphoria, major depression, mood altered, negative thoughts, suicide attempt, suicidal ideation) have been See full prescribing information for complete boxed warning. reported. Immediate medical evaluation is recommended for severe depressive disorders. (5.5) • Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of • Decreases in bone mineral density (BMD): Consider monitoring nucleoside analogs, including tenofovir disoproxil BMD in patients with a history of pathologic fracture -

Comparable Efficacy with Entecavir Monotherapy and Tenofovir
BMJ Open Gastroenterol: first published as 10.1136/bmjgast-2015-000030 on 31 December 2015. Downloaded from Hepatology Comparable efficacy with entecavir monotherapy and tenofovir–entecavir combination in chronic hepatitis B patients Sumbella Baqai,1 James Proudfoot,2 Ronghui Xu,3,4 Steve Kane,5 Margaret Clark,6 Robert Gish,7,8,9,10,11 To cite: Baqai S, Proudfoot J, ABSTRACT et al Summary box Xu R, . Comparable Objectives: The long-term goal for chronic hepatitis B efficacy with entecavir patients is to maintain viral suppression in order to monotherapy and tenofovir– What is already known about this subject? reduce disease progression risk. Because patients with entecavir combination in ▸ Finding effective therapy for patients with chronic hepatitis B patients. previous treatment failure may have multiple viral chronic hepatitis B virus (HBV) infection who BMJ Open Gastro 2015;2: resistance mutations, finding effective therapy is e000030. doi:10.1136/ challenging. Because recent studies have shown that have had previous treatment failure, many of bmjgast-2015-000030 the combination of entecavir and tenofovir is effective whom have multiple viral resistance mutations, in achieving virological response in many patients with is a challenge. prior treatment failure and multiple drug resistance ▸ Several recent studies have shown that combin- ation therapy with entecavir (ETV) and tenofovir Received 27 January 2015 mutations, we compared outcomes with this Revised 22 April 2015 combination versus monotherapy. (TDF) is effective in achieving virological by copyright. Accepted 30 April 2015 Methods: With a retrospective chart review we response in the majority of patients with prior compared results in 35 patients with previous treatment treatment failure and multiple drug resistance failure treated with the entecavir-tenofovir combination to mutations. -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m. -

Varicella (Chickenpox): Questions and Answers Q&A Information About the Disease and Vaccines
Varicella (Chickenpox): Questions and Answers Q&A information about the disease and vaccines What causes chickenpox? more common in infants, adults, and people with Chickenpox is caused by a virus, the varicella-zoster weakened immune systems. virus. How do I know if my child has chickenpox? How does chickenpox spread? Usually chickenpox can be diagnosed by disease his- Chickenpox spreads from person to person by direct tory and appearance alone. Adults who need to contact or through the air by coughing or sneezing. know if they’ve had chickenpox in the past can have It is highly contagious. It can also be spread through this determined by a laboratory test. Chickenpox is direct contact with the fluid from a blister of a per- much less common now than it was before a vaccine son infected with chickenpox, or from direct contact became available, so parents, doctors, and nurses with a sore from a person with shingles. are less familiar with it. It may be necessary to perform laboratory testing for children to confirm chickenpox. How long does it take to show signs of chickenpox after being exposed? How long is a person with chickenpox contagious? It takes from 10 to 21 days to develop symptoms after Patients with chickenpox are contagious for 1–2 days being exposed to a person infected with chickenpox. before the rash appears and continue to be conta- The usual time period is 14–16 days. gious through the first 4–5 days or until all the blisters are crusted over. What are the symptoms of chickenpox? Is there a treatment for chickenpox? The most common symptoms of chickenpox are rash, fever, coughing, fussiness, headache, and loss of appe- Most cases of chickenpox in otherwise healthy children tite. -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal -

The Study of Thimerosal and Autism
The Study of Thimerosal and Autism Documentation and Codebook for the Child Vaccination Histories File: Cambridge, MA Lexington, MA Hadley, MA Bethesda, MD September 3, 2010 Chicago, IL Prepared for Frank DeStefano National Immunization Program Centers for Disease Control and Prevention Atlanta, GA 30329 Prepared by Cristofer Price Yeqin He Abt Associates Inc. Suite 800 North 4550 Montgomery Avenue Bethesda, MD 20814-3343 This documentation was prepared by Cristofer Price and Yeqin He of Abt Associates Inc. for the Immunization Safety Office (ISO) of the Centers for Disease Control and Prevention (CDC) Atlanta, GA 30333. Questions about the documentation, substantive questions, or technical issues regarding the data file should be directed to the CDC ISO, MS D26, 1600 Clifton Road, Atlanta, Georgia 30333 (404-639-8256). Suggested Citation for Study of Thimerosal and Autism Public Use Data Set: Price, C.S., He, Y. (2010) The Study of Thimerosal and Autism: Data Set Documentation. Bethesda MD: Abt Associates, Inc; Prepared for the National Immunization Program Centers for Disease Control and Prevention Atlanta, GA Abt Associates Inc. 1 Documentation and Codebook for the Child Vaccination Histories File 1. Introduction to the Child Vaccination Histories File............................................... 2 2. File Formats and Variable Descriptions................................................................... 4 1. Introduction to the Child Vaccination Histories File The Child Vaccination Histories File contains the data that were used to construct the measures of early childhood (postnatal) exposure to ethylmercury from thimerosal- containing vaccines and immune globulin preparations received by the study children during the age range spanning birth to 20 months. The Child Vaccination Histories File is provided with the public use data in order to make the calculation of exposure amounts transparent, and so that analysts have the potential to calculate alternative measures of exposure1. -

Gsk Vaccines in 2010
GSK VACCINES IN 2010 Thomas Breuer, MD, MSc Senior Vice President Head of Global Vaccines Development GSK Biologicals Vaccines business characteristics Few global players and high barriers to entry – Complex manufacturing – Large scale investment Long product life cycles – Complex intellectual property High probability of R&D success – 70% post-POC New technology/novel products Better pricing for newer vaccines – HPV vaccines (Cervarix, Gardasil) – Pneumococcal vaccines (Synflorix, Prevnar-13) Operating margin comparable to pharmaceutical products Heightened awareness New markets 2 Research & development timelines Identify Produce Pre-Clinical Proof of Registration/ Phase I Phase II Phase III File Antigens Antigens Testing Concept Post Marketing Research (inc. Immunology) Pre-Clinical Development (inc. Formulation Science) Clinical Development (inc. Post Marketing Surveillance) Transfer Process to Manufacturing Build Facility x x Up to $10-20M Up to $50-100M $500M - $1B x x x 1-10 yrs 2-3 yrs 2-4 yrs > 1 yr GSK vaccines business 2009 sales £3.7 billion (+30%) +19% CAGR excl. H1N1 Vaccines represent 13% since 2005 of total GSK sales Sale s (£m) 4000 3500 Recent approvals: 3000 US: Cervarix 2500 EU: Synflorix 2000 Pandemic: Pandemrix; Arepanrix 1500 1000 500 0 2005 2006 2007 2008 2009 Increased Emerging Market presence Growth rate is CER 5 GSK vaccines: fastest growing part of GSK in 2009 2009 Sales Share Growth (CER) Respiratory £ 6,977m 25% +5% Consumer £ 4,654m 16% +7% Anti-virals £ 4,150m 15% +12% Vaccines £ 3,706m 13% +30% CV & Urogenital -
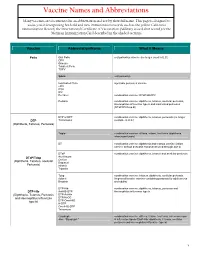
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus, -

Visible Spectrophotometric Determination of Famciclovir in Bulk and Pharmaceutical Dosage Formulations
Asian Journal of Chemistry Vol. 19, No. 5 (2007), 3617-3620 Visible Spectrophotometric Determination of Famciclovir in Bulk and Pharmaceutical Dosage Formulations SYED NIZAMUDDIN*, DIVAKAR GOLI, Y.N. MANOHARA† and M.C. RAVI Department of Pharmaceutical Analysis Acharya & B.M. Reddy College of Pharmacy, Bangalore-560 090, India Tel: (91)9845121221 (M); E-mail: [email protected] Two simple colorimetric methods for the estimation of famciclovir in pharmaceutical dosage forms. In method A famciclovir undergoes complexation with SNP and to form coloured chromogen exhibiting absorption maximum at 428 nm and obeyed Beer's law in the concentration range of 2-12 µg/mL. In the method B based on the formation of an ion pair with bromocresol green in acidic medium and the subse- quent extraction of the ion pair in chloroform. The yellow coloured ion pair showed an absorption maximum at 421 nm with apparent molar absorptivity. The proposed methods gave reproducible results for the estimation of famciclovir from its pharmaceutical formulation. Key Words: Spectrophotometric analysis, Famciclovir. INTRODUCTION Famciclovir is chemically 2-[2-(2-amino-9H-purin-9-yl)ethyl] trim- ethylene diacetate1,2 is an acyclic guanine nucleoside analogue. It is a new generation antiviral drug which is active in vitro and in vivo against herpes simplex virus types 1 and 2 and against varicella-zoster virus3-6. It is not official in any Pharmacopoeia. A few analytical methods have been re- ported for its quantitative estimation in pharmaceutical formulations that include estimation in plasma and urine by HPLC7 and UV8 spectrophoto- metric estimation in methanol. The present work describes two spectro- photometric methods for the estimation of famciclovir in its formulations.