Lectures on Mechanics
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Particle-On-A-Ring” Suppose a Diatomic Molecule Rotates in Such a Way That the Vibration of the Bond Is Unaffected by the Rotation
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015 Lecture 17 2/25/15 Recommended Reading Atkins & DePaula 9.5 A. Background in Rotational Mechanics Two masses m1 and m2 connected by a weightless rigid “bond” of length r rotates with angular velocity rv where v is the linear velocity. As with vibrations we can render this rotation motion in simpler form by fixing one end of the bond to the origin terminating the other end with reduced mass , and allowing the reduced mass to rotate freely For two masses m1 and m2 separated by r the center of mass (CoM) is defined by the condition mr11 mr 2 2 (17.1) where m2,1 rr1,2 mm12 (17.2) Figure 17.1: Location of center of mass (CoM0 for two masses separated by a distance r. The moment of inertia I plays the role in the rotational energy that is played by mass in translational energy. The moment of inertia is 22 I mr11 mr 2 2 (17.3) Putting 17.3 into 17.4 we obtain I r 2 (17.4) mm where 12 . mm12 We can express the rotational kinetic energy K in terms of the angular momentum. The angular momentum is defined in terms of the cross product between the position vector r and the linear momentum vector p: Lrp (17.5) prprvrsin where the angle between p and r is ninety degrees for circular motion. As a cross product, the angular momentum vector L is perpendicular to the plane defined by the r and p vectors as shown in Figure 17.2 We can use equation 17.6 to obtain an expression for the kinetic energy in terms of the angular momentum L: Figure 17.2: The angular momentum L is a cross product of the position r vector and the linear momentum p=mv vector. -

Rotational Spectroscopy
Applied Spectroscopy Rotational Spectroscopy Recommended Reading: 1. Banwell and McCash: Chapter 2 2. Atkins: Chapter 16, sections 4 - 8 Aims In this section you will be introduced to 1) Rotational Energy Levels (term values) for diatomic molecules and linear polyatomic molecules 2) The rigid rotor approximation 3) The effects of centrifugal distortion on the energy levels 4) The Principle Moments of Inertia of a molecule. 5) Definitions of symmetric , spherical and asymmetric top molecules. 6) Experimental methods for measuring the pure rotational spectrum of a molecule Microwave Spectroscopy - Rotation of Molecules Microwave Spectroscopy is concerned with transitions between rotational energy levels in molecules. Definition d Electric Dipole: p = q.d +q -q p H Most heteronuclear molecules possess Cl a permanent dipole moment -q +q e.g HCl, NO, CO, H2O... p Molecules can interact with electromagnetic radiation, absorbing or emitting a photon of frequency ω, if they possess an electric dipole moment p, oscillating at the same frequency Gross Selection Rule: A molecule has a rotational spectrum only if it has a permanent dipole moment. Rotating molecule _ _ + + t _ + _ + dipole momentp dipole Homonuclear molecules (e.g. O2, H2, Cl2, Br2…. do not have a permanent dipole moment and therefore do not have a microwave spectrum! General features of rotating systems m Linear velocity v angular velocity v = distance ω = radians O r time time v = ω × r Moment of Inertia I = mr2. A molecule can have three different moments of inertia IA, IB and IC about orthogonal axes a, b and c. 2 I = ∑miri i R Note how ri is defined, it is the perpendicular distance from axis of rotation ri Rigid Diatomic Rotors ro IB = Ic, and IA = 0. -

Characterization of Exact Lumpability for Vector Fields on Smooth Manifolds
Preprint. Final version in: Differ.Geom.Appl. 48 (2016) 46-60 doi: 10.1016/j.difgeo.2016.06.001 Characterization of Exact Lumpability for Vector Fields on Smooth Manifolds Leonhard Horstmeyer∗ † Fatihcan M. Atay‡ Abstract We characterize the exact lumpability of smooth vector fields on smooth man- ifolds. We derive necessary and sufficient conditions for lumpability and express them from four different perspectives, thus simplifying and generalizing various re- sults from the literature that exist for Euclidean spaces. We introduce a partial connection on the pullback bundle that is related to the Bott connection and be- haves like a Lie derivative. The lumping conditions are formulated in terms of the differential of the lumping map, its covariant derivative with respect to the con- nection and their respective kernels. Some examples are discussed to illustrate the theory. PACS numbers: 02.40.-k, 02.30.Hq, 02.40.Hw AMS classification scheme numbers: 37C10, 34C40, 58A30, 53B05, 34A05 Keywords: lumping, aggregation, dimensional reduction, Bott connection 1 Introduction Dimensional reduction is an important aspect in the study of smooth dynamical systems and in particular in modeling with ordinary differential equations (ODEs). Often a reduction can elucidate key mechanisms, find decoupled subsystems, reveal conserved quantities, make the problem computationally tractable, or rid it from redundancies. A dimensional reduction by which micro state variables are aggregated into macro state variables also goes by the name of lumping. Starting from a micro state dynamics, this arXiv:1607.01237v2 [math.DG] 6 Jul 2016 aggregation induces a lumped dynamics on the macro state space. Whenever a non- trivial lumping, one that is neither the identity nor maps to a single point, confers the ∗Max Planck Institute for Mathematics in the Sciences, Inselstraße 22, 04103 Leipzig, Germany. -

Lecture 6: 3D Rigid Rotor, Spherical Harmonics, Angular Momentum
Lecture 6: 3D Rigid Rotor, Spherical Harmonics, Angular Momentum We can now extend the Rigid Rotor problem to a rotation in 3D, corre- sponding to motion on the surface of a sphere of radius R. The Hamiltonian operator in this case is derived from the Laplacian in spherical polar coordi- nates given as ∂2 ∂2 ∂2 ∂2 2 ∂ 1 1 ∂2 1 ∂ ∂ ∇2 = + + = + + + sin θ ∂x2 ∂y2 ∂z2 ∂r2 r ∂r r2 sin2 θ ∂φ2 sin θ ∂θ ∂θ For constant radius the first two terms are zero and we have 2 2 1 ∂2 1 ∂ ∂ Hˆ (θ, φ) = − ~ ∇2 = − ~ + sin θ 2m 2mR2 sin2 θ ∂φ2 sin θ ∂θ ∂θ We also note that Lˆ2 Hˆ = 2I where the operator for squared angular momentum is given by 1 ∂2 1 ∂ ∂ Lˆ2 = − 2 + sin θ ~ sin2 θ ∂φ2 sin θ ∂θ ∂θ The Schr¨odingerequation is given by 2 1 ∂2ψ(θ, φ) 1 ∂ ∂ψ(θ, φ) − ~ + sin θ = Eψ(θ, φ) 2mR2 sin2 θ ∂φ2 sin θ ∂θ ∂θ The wavefunctions are quantized in 2 directions corresponding to θ and φ. It is possible to derive the solutions, but we will not do it here. The solutions are denoted by Yl,ml (θ, φ) and are called spherical harmonics. The quantum 1 numbers take values l = 0, 1, 2, 3, .... and ml = 0, ±1, ±2, ... ± l. The energy depends only on l and is given by 2 E = l(l + 1) ~ 2I The first few spherical harmonics are given by r 1 Y = 0,0 4π r 3 Y = cos(θ) 1,0 4π r 3 Y = sin(θ)e±iφ 1,±1 8π r 5 Y = (3 cos2 θ − 1) 2,0 16π r 15 Y = cos θ sin θe±iφ 2,±1 8π r 15 Y = sin2 θe±2iφ 2,±2 32π These spherical harmonics are related to atomic orbitals in the H-atom. -

A 3D Model of the Rigid Rotor Supported by Journal Bearings)
A 3D MODEL OF THE RIGID ROTOR SUPPORTED BY JOURNAL BEARINGS) Jiří TŮMA1, Radim KLEČKA2, Jaromír ŠKUTA3 and Jiří ŠIMEK4 1 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 2 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 3 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 4 Techlab s.r.o., Sokolovská 207, 190 00 Praha 9, [email protected] Keywords: up to 7 keywords (10 pt) 1. Introduction It is known that the journal bearing with an oil film becomes instable if the rotor rotation speed crosses a certain value, which is called the Bently-Muszynska threshold [1]. To prevent the rotor instability, the active control can be employed. The arrangement of proximity probes and piezoactuators in a rotor system is shown in figure 1. It is assumed that the bushings (carrier ring), inserted into pedestals with clearance, is a movable part in two perpendicular directions while rotor is rotating. Im(r) Bushing Proximity probes Re(r) Ω Re(u) Journal Piezoactuators Im(r) Fig. 1. Journal coordinates and arrangement The research work supported by the GAČR (project no. 101/07/1345) is aimed at the design of the journal bearing active control based on the carrier ring position manipulation by the piezoactuators according to the proximity probe signals, which are a part of the closed loop including a controller. The effect of the feedback on the rotor stability is analyzed by [5]. The test stand of the TECHLAB design [1] is shown in figure 2. -
Rotational Spectroscopy
Rotational spectroscopy - Involve transitions between rotational states of the molecules (gaseous state!) - Energy difference between rotational levels of molecules has the same order of magnitude with microwave energy - Rotational spectroscopy is called pure rotational spectroscopy, to distinguish it from roto-vibrational spectroscopy (the molecule changes its state of vibration and rotation simultaneously) and vibronic spectroscopy (the molecule changes its electronic state and vibrational state simultaneously) Molecules do not rotate around an arbitrary axis! Generally, the rotation is around the mass center of the molecule. The rotational axis must allow the conservation of M R α pα const kinetic angular momentum. α Rotational spectroscopy Rotation of diatomic molecule - Classical description Diatomic molecule = a system formed by 2 different masses linked together with a rigid connector (rigid rotor = the bond length is assumed to be fixed!). The system rotation around the mass center is equivalent with the rotation of a particle with the mass μ (reduced mass) around the center of mass. 2 2 2 2 m1m2 2 The moment of inertia: I miri m1r1 m2r2 R R i m1 m2 Moment of inertia (I) is the rotational equivalent of mass (m). Angular velocity () is the equivalent of linear velocity (v). Er → rotational kinetic energy L = I → angular momentum mv 2 p2 Iω2 L2 E E c 2 2m r 2 2I Quantum rotation: The diatomic rigid rotor The rigid rotor represents the quantum mechanical “particle on a sphere” problem: Rotational energy is purely -

Lorentzian Cobordisms, Compact Horizons and the Generic Condition
Lorentzian Cobordisms, Compact Horizons and the Generic Condition Eric Larsson Master of Science Thesis Stockholm, Sweden 2014 Lorentzian Cobordisms, Compact Horizons and the Generic Condition Eric Larsson Master’s Thesis in Mathematics (30 ECTS credits) Degree programme in Engineering Physics (300 credits) Royal Institute of Technology year 2014 Supervisor at KTH was Mattias Dahl Examiner was Mattias Dahl TRITA-MAT-E 2014:29 ISRN-KTH/MAT/E--14/29--SE Royal Institute of Technology School of Engineering Sciences KTH SCI SE-100 44 Stockholm, Sweden URL: www.kth.se/sci iii Abstract We consider the problem of determining which conditions are necessary for cobordisms to admit Lorentzian metrics with certain properties. In particu- lar, we prove a result originally due to Tipler without a smoothness hypothe- sis necessary in the original proof. In doing this, we prove that compact hori- zons in a smooth spacetime satisfying the null energy condition are smooth. We also prove that the ”generic condition” is indeed generic in the set of Lorentzian metrics on a given manifold. Acknowledgements I would like to thank my advisor Mattias Dahl for invaluable advice and en- couragement. Thanks also to Hans Ringström. Special thanks to Marc Nardmann for feedback on Chapter 2. Contents Contents iv 1 Lorentzian cobordisms 1 1.1 Existence of Lorentzian cobordisms . 2 1.2 Lorentzian cobordisms and causality . 4 1.3 Lorentzian cobordisms and energy conditions . 8 1.3.1 C 2 null hypersurfaces . 9 1.3.1.1 The null Weingarten map . 9 1.3.1.2 Generator flow on C 2 null hypersurfaces . -

The Holomorphic Φ-Sectional Curvature of Tangent Sphere Bundles with Sasakian Structures
ANALELE S¸TIINT¸IFICE ALE UNIVERSITAT¸II˘ \AL.I. CUZA" DIN IAS¸I (S.N.) MATEMATICA,˘ Tomul LVII, 2011, Supliment DOI: 10.2478/v10157-011-0004-5 THE HOLOMORPHIC '-SECTIONAL CURVATURE OF TANGENT SPHERE BUNDLES WITH SASAKIAN STRUCTURES BY S. L. DRUT¸ A-ROMANIUC˘ and V. OPROIU Abstract. We study the holomorphic '-sectional curvature of the natural diagonal tangent sphere bundles with Sasakian structures, determined by Drut¸a-Romaniuc˘ and Oproiu. After finding the explicit expressions for the components of the curvature tensor field and of the curvature tensor field corresponding to the Sasakian space forms, we find that there are no tangent sphere bundles of natural diagonal lift type of constant holomorphic '-sectional curvature. Mathematics Subject Classification 2000: 53C05, 53C15, 53C55. Key words: natural lift, tangent sphere bundle, Sasakian structure, holomorphic '-sectional curvature. 1. Introduction The geometry of the tangent sphere bundles of constant radius r, endowed with the metrics induced by some Riemannian metrics from the ambi- ent tangent bundle, was studied by authors like Abbassi, Boeckx, Cal- varuso, Kowalski, Munteanu, Park, Sekigawa, Sekizawa (see [1], [2], [4]-[7], [9], [12]-[14], [16]). In the most part of the papers, such as [4], [5] and [16], the metric considered on the tangent bundle TM was the Sasaki metric, but Boeckx noticed that the unit tangent bundle equipped with the induced Cheeger-Gromoll metric is isometric to the tangent sphere bun- dle T p1 M with the induced Sasaki metric. Then, in 2000, Kowalski and 2 Sekizawa showed how the geometry of the tangent sphere bundles depends on the radius (see [10]). -
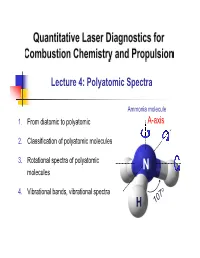
Lecture 4: Polyatomic Spectra
Lecture 4: Polyatomic Spectra Ammonia molecule 1. From diatomic to polyatomic A-axis 2. Classification of polyatomic molecules 3. Rotational spectra of polyatomic N molecules 4. Vibrational bands, vibrational spectra H 1. From diatomic to polyatomic Rotation – Diatomics Recall: For diatomic molecules Energy: FJ ,cm1 BJJ 1 DJ 2 J 12 R.R. Centrifugal distortion constant h Rotational constant: B,cm1 8 2 Ic Selection Rule: J ' J"1 J 1 3 Line position: J "1J " 2BJ"1 4D J"1 Notes: 2 1. D is small, i.e., D / B 4B / vib 1 2 2 D B 1.7 6 2. E.g., for NO, 4 4 310 B NO e 1900 → Even @ J=60, D / B J 2 ~ 0.01 What about polyatomics (≥3 atoms)? 2 1. From diatomic to polyatomic 3D-body rotation B . Convention: A A-axis is the “unique” or “figure” axis, along which lies the molecule’s C defining symmetry . 3 principal axes (orthogonal): A, B, C . 3 principal moments of inertia: IA, IB, IC . Molecules are classified in terms of the relative values of IA, IB, IC 3 2. Classification of polyatomic molecules Types of molecules Linear Symmetric Asymmetric Type Spherical Tops Molecules Tops Rotors Relative IB=IC≠IA magnitudes IB=IC; IA≈0* IA=IB=IC IA≠IB≠IC IA≠0 of IA,B,C NH CO2 3 H2O CH4 C2H2 CH F Examples 3 Acetylene NO2 OCS BCl3 Carbon Boron oxysulfide trichloride Relatively simple No dipole moment Largest category Not microwave active Most complex *Actually finite, but quantized momentum means it is in lowest state of rotation 4 2. -

On Manifolds of Negative Curvature, Geodesic Flow, and Ergodicity
ON MANIFOLDS OF NEGATIVE CURVATURE, GEODESIC FLOW, AND ERGODICITY CLAIRE VALVA Abstract. We discuss geodesic flow on manifolds of negative sectional curva- ture. We find that geodesic flow is ergodic on the tangent bundle of a manifold, and present the proof for both n = 2 on surfaces and general n. Contents 1. Introduction 1 2. Riemannian Manifolds 1 2.1. Geodesic Flow 2 2.2. Horospheres and Horocycle Flows 3 2.3. Curvature 4 2.4. Jacobi Fields 4 3. Anosov Flows 4 4. Ergodicity 5 5. Surfaces of Negative Curvature 6 5.1. Fuchsian Groups and Hyperbolic Surfaces 6 6. Geodesic Flow on Hyperbolic Surfaces 7 7. The Ergodicity of Geodesic Flow on Compact Manifolds of Negative Sectional Curvature 8 7.1. Foliations and Absolute Continuity 9 7.2. Proof of Ergodicity 12 Acknowledgments 14 References 14 1. Introduction We want to understand the behavior of geodesic flow on a manifold M of constant negative curvature. If we consider a vector in the unit tangent bundle of M, where does that vector go (or not go) when translated along its unique geodesic path. In a sense, we will show that the vector goes \everywhere," or that the vector visits a full measure subset of T 1M. 2. Riemannian Manifolds We first introduce some of the initial definitions and concepts that allow us to understand Riemannian manifolds. Date: August 2019. 1 2 CLAIRE VALVA Definition 2.1. If M is a differentiable manifold and α :(−, ) ! M is a dif- ferentiable curve, where α(0) = p 2 M, then the tangent vector to the curve α at t = 0 is a function α0(0) : D ! R, where d(f ◦ α) α0(0)f = j dt t=0 for f 2 D, where D is the set of functions on M that are differentiable at p. -

Quantum Control of Molecular Rotation Is Challenging and Promising at the Same Time
Quantum control of molecular rotation Christiane P. Koch,∗ Mikhail Lemeshko,† Dominique Sugny‡ October 10, 2019 Abstract The angular momentum of molecules, or, equivalently, their rotation in three-dimensional space, is ideally suited for quantum control. Molecular angular momentum is naturally quantized, time evolution is governed by a well-known Hamiltonian with only a few accurately known parameters, and transitions between rota- tional levels can be driven by external fields from various parts of the electromagnetic spectrum. Control over the rotational motion can be exerted in one-, two- and many-body scenarios, thereby allowing to probe Anderson localization, target stereoselectivity of bimolecular reactions, or encode quantum information, to name just a few examples. The corresponding approaches to quantum control are pursued within sepa- rate, and typically disjoint, subfields of physics, including ultrafast science, cold collisions, ultracold gases, quantum information science, and condensed matter physics. It is the purpose of this review to present the various control phenomena, which all rely on the same underlying physics, within a unified framework. To this end, we recall the Hamiltonian for free rotations, assuming the rigid rotor approximation to be valid, and summarize the different ways for a rotor to interact with external electromagnetic fields. These inter- actions can be exploited for control — from achieving alignment, orientation, or laser cooling in a one-body framework, steering bimolecular collisions, or realizing a quantum computer or quantum simulator in the many-body setting. 1 Introduction Molecules, unlike atoms, are extended objects that possess a number of different types of motion. In particular, the geometric arrangement of their constituent atoms endows molecules with the basic capability to rotate in three-dimensional space. -

Chapter 7. a Quantum Mechanical Model for the Vibration and Rotation of Molecules
Chapter 7. A Quantum Mechanical Model for the Vibration and Rotation of Molecules Harmonic oscillator: Hooke’s law: F kx, x is displacement Harmonic potential: 1 2 V (x) Fdx kx 2 k is force constant: d 2V k (curvature of V at equilibrium) 2 dx x0 Newton’s equation: d 2x m F kx (diff. eqn) dt 2 Solutions: x = Asint, (position) 1/ 2 (k/m) (vibrational frequency) 1 Verify: d 2 Asin t d lhs m mA cost mA2 sin t dt 2 dt k m Asin t kx rhs m Momentum: dx p m mAcost dt Energy: p2 E V 2m (mAcost)2 1 k(Asin t)2 2m 2 m 2 A2 (cost)2 (sin t)2 2 m 2 A2 kA2 2 2 When t = 0, x = 0 and p = p = mA max When t = , p = 0 and x = x = A max Energy conservation is maintained by oscillation between kinetic and potential energies. 2 Schrödinger equation for harmonic oscillator: 2 2 d 1 2 2 kx E 2m dx 2 Energy is quantized: 1 E 0, 1, ... 2 where the vibrational frequency 1/ 2 k m Restriction of motion leads to uncertainty in x and p, and quantization of energy. Wavefunctions: y2 / 2 (x) N H (y)e where 1/ 4 mk y x, 2 Normalization factor 3 1/ 2 N 2! H (y) is the Hermite polynomial H0 (y) 1 H1(y) 2y 2 H2 (y) 4y 2 ... H1 2yH 2H1 (recursion relation) Let’s verify for the ground state 2 x2 / 2 0 (x) N0e 2 2 d 1 2 2 x 2 / 2 lhs N0 2 kx e 2m dx 2 2 d 2 x 2 / 2 2 1 2 2 x 2 / 2 N0 e x kx e 2m dx 2 2 2 x 2 / 2 2 2 2 x 2 / 2 2 1 2 2 x 2 / 2 N0 e x e kx e 2m 2 2 4 2 2 k 2 2 2 x / 2 N0 x e 2 2m 2m Note that 4 1/ 4 mk 2 It is not difficult to prove that the first term is zero, and the second term as / 2.