Tese Rita Bento
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Trends of Aquatic Alien Species Invasions in Ukraine
Aquatic Invasions (2007) Volume 2, Issue 3: 215-242 doi: http://dx.doi.org/10.3391/ai.2007.2.3.8 Open Access © 2007 The Author(s) Journal compilation © 2007 REABIC Research Article Trends of aquatic alien species invasions in Ukraine Boris Alexandrov1*, Alexandr Boltachev2, Taras Kharchenko3, Artiom Lyashenko3, Mikhail Son1, Piotr Tsarenko4 and Valeriy Zhukinsky3 1Odessa Branch, Institute of Biology of the Southern Seas, National Academy of Sciences of Ukraine (NASU); 37, Pushkinska St, 65125 Odessa, Ukraine 2Institute of Biology of the Southern Seas NASU; 2, Nakhimova avenue, 99011 Sevastopol, Ukraine 3Institute of Hydrobiology NASU; 12, Geroyiv Stalingrada avenue, 04210 Kiyv, Ukraine 4Institute of Botany NASU; 2, Tereschenkivska St, 01601 Kiyv, Ukraine E-mail: [email protected] (BA), [email protected] (AB), [email protected] (TK, AL), [email protected] (PT) *Corresponding author Received: 13 November 2006 / Accepted: 2 August 2007 Abstract This review is a first attempt to summarize data on the records and distribution of 240 alien species in fresh water, brackish water and marine water areas of Ukraine, from unicellular algae up to fish. A checklist of alien species with their taxonomy, synonymy and with a complete bibliography of their first records is presented. Analysis of the main trends of alien species introduction, present ecological status, origin and pathways is considered. Key words: alien species, ballast water, Black Sea, distribution, invasion, Sea of Azov introduction of plants and animals to new areas Introduction increased over the ages. From the beginning of the 19th century, due to The range of organisms of different taxonomic rising technical progress, the influence of man groups varies with time, which can be attributed on nature has increased in geometrical to general processes of phylogenesis, to changes progression, gradually becoming comparable in in the contours of land and sea, forest and dimensions to climate impact. -

PROJECT REPORT Expedition Dates: 6 – 12 October 2013 Report Published: April 2014
PROJECT REPORT Expedition dates: 6 – 12 October 2013 Report published: April 2014 Underwater pioneers: studying & protecting the unique coral reefs of the Musandam peninsula, Oman. n e k t i A n i v l e K ) c ( e g a m i r e v o C BEST BEST FOR TOP BEST WILDLIFE BEST IN ENVIRONMENT TOP HOLIDAY VOLUNTEERING GREEN-MINDED RESPONSIBLE VOLUNTEERING SUSTAINABLE AWARD FOR NATURE ORGANISATION TRAVELLERS HOLIDAY HOLIDAY TRAVEL Germany Germany UK UK UK UK USA EXPEDITION REPORT Underwater pioneers: studying & protecting the unique coral reefs of the Musandam peninsula, Oman. Expedition dates: 6 – 12 October 2013 Report published: February 2014 Authors: Jean-Luc Solandt Marine Conservation Society Matthias Hammer (editor) Biosphere Expeditions 1 © Biosphere Expeditions, an international not-for-profit conservation organisation – www.biosphere-expeditions.org Member of the United Nations Environment Programme's Governing Council & Global Ministerial Environment Forum Member of the International Union for the Conservation of Nature Abstract Coral reefs are important biodiversity hotspots that not only function as a crucial habitat for a multitude of organisms, but also provide human populations with an array of goods and services, such as food and coastal protection. Despite this, coral reefs are under threat worldwide from direct or indirect anthropogenic impacts, such as pollution, overexploitation and climate change. The coral reefs of the Musandam peninsula (Oman), situated on the Arabian Peninsula in the Strait of Hormuz, endure extreme conditions such as high salinity and temperatures, existing – indeed thriving – in what would be considered marginal and highly challenging environments for corals in other parts of the world. -

CAESIONIDAE Fusiliers by K.E
click for previous page Perciformes: Percoidei: Caesonidae 2919 CAESIONIDAE Fusiliers by K.E. Carpenter iagnostic characters: Oblong to fusiform, moderately compressed, medium-sized to small (to about D50 cm) lutjanoid fishes; longitudinal axis from tip of snout to middle of caudal fin passing through centre of eye. Eye moderately large, its diameter longer than snout length. Mouth small and highly protrusible; 1 or 2 finger-like postmaxillary processes on dorsoposterior surface of premaxilla (Figs 1 and 2); angle of jaw oblique, about 40° to horizontal. Dentition variously reduced; small or minute conical teeth; premaxillae, vomer, and palatines with or without teeth. Caudal fin deeply forked. Margin of dorsal and anal fins more or less evenly sloping; third or fourth dorsal-fin spines longest; second or third anal-fin spines longest, remaining spines and rays gradually decreasing in length (except in Dipterygonotus with dorsal fin profile not evenly sloping, last IV-V dorsal-fin spines small and nearly separate, connected only at their bases by membrane, and dorsal-fin rays much longer than these spines). Dorsal fin with X to XV slender weak spines and 8 to 22 soft rays; anal fin with III spines and 9 to 13 soft rays;pelvicfins with I spine and 5 soft rays; pectoral fins with 16 to 24 rays; caudal fin distinctly forked, with pointed lobes. Branchiostegal rays 7. Scales moderate to small, weakly ctenoid; lateral-line scales 45 to 88; scale rows on body running horizontally; dorsal and anal fins with scales except for Gymnocaesio gymnoptera and Dipterygonotus balteatus. Ascending premaxillary process a separate ossification from premaxilla; ethmo-maxillary ligament absent; a separate A1’ section of the adductor mandibulae which originates on the subocular shelf. -

Mantas, Dolphins and Coral Reefs – a Maldives Cruise
Mantas, Dolphins and Coral Reefs – A Maldives Cruise Naturetrek Tour Report 1 - 10 March 2018 Crabs by Pat Dean Hermit Crab by Pat Dean Risso’s Dolphin by Pat Dean Titan Triggerfish by Jenny Willsher Report compiled by Jenny Willsher Images courtesy of Pat Dean & Jenny Willsher Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Mantas, Dolphins and Coral Reefs – A Maldives Cruise Tour participants: Dr Chas Anderson (cruise leader) & Jenny Willsher (leader) with 13 Naturetrek clients Introduction For centuries the Maldives was a place to avoid if you were a seafarer due to its treacherous reefs, and this may have contributed to its largely unspoilt beauty. Now those very same reefs attract many visitors to experience the amazing diversity of marine life that it offers. Sharks and Scorpion fish, Octopus, Lionfish, Turtles and legions of multi-coloured fish of all shapes and sizes are to be found here! Add to that an exciting variety of cetaceans and you have a wildlife paradise. Despite the frustrating hiccoughs experienced by various members of the group in their travels, due to the snowy weather in the UK, we had a successful week in and around this intriguing chain of coral islands. After a brief stay in the lovely Bandos Island Resort (very brief for Pat and Stuart!), which gave us time for some snorkel practice, we boarded the MV Theia, our base for the next week. We soon settled into the daily routine of early morning and evening snorkels, daytimes searching for cetaceans or relaxing, and evening talks by Chas, our local Maldives expert. -

National Reports (Term of Reference A) Presented at the 46Th Meeting of the ICES Working Group on Introductions and Transfers Of
National Reports (Term of Reference a) Presented at the 46th meeting of the ICES Working Group on Introductions and Transfers of Marine Organisms, held in Gdynia, Poland from 4 to 6 March 2020. Arranged Alphabetically by Country Compiled by Cynthia H McKenzie, Chair, WGITMO CANADA …………………………………………………………………… …. 2 DENMARK ………………………………………………………………………. 10 FINLAND ………………………………………………………………………. 18 FRANCE ………………………………………………………………………. 21 GERMANY ………………………………………………………………………. 33 GREECE ………………………………………………………………………. 46 ICELAND ………………………………………………………………………. 54 ITALY ………………………………………………………………………. 58 LITHUANIA ………………………………………………………………………. 73 NETHERLANDS …………………………………………………………………. 75 NORWAY ………………………………………………………………………. 77 POLAND ………………………………………………………………………. 86 PORTUGAL ………………………………………………………………………. 89 SWEDEN ………………………………………………………………………. 99 UNITED KINGDOM ……………………………………………………………. 104 UNITED STATES …………………………………………………………………. 119 1 CANADA National Report for Canada 2019 Report Prepared By: Cynthia McKenzie, Fisheries and Oceans Canada, Newfoundland and Labrador Region: [email protected]; Contributions By: Nathalie Simard, Fisheries and Oceans Canada, Quebec Region: [email protected]; Kimberly Howland, Fisheries and Oceans Canada, Central and Arctic Region: [email protected]; Renée Bernier and Chantal Coomber, Fisheries and Oceans Canada, Gulf Region: renee.bernier@dfo- mpo.gc.ca, [email protected]; Angelica Silva, Fisheries and Oceans Canada, Maritimes Region: [email protected] Overview: NEW or SPREAD -

The Global Trade in Marine Ornamental Species
From Ocean to Aquarium The global trade in marine ornamental species Colette Wabnitz, Michelle Taylor, Edmund Green and Tries Razak From Ocean to Aquarium The global trade in marine ornamental species Colette Wabnitz, Michelle Taylor, Edmund Green and Tries Razak ACKNOWLEDGEMENTS UNEP World Conservation This report would not have been The authors would like to thank Helen Monitoring Centre possible without the participation of Corrigan for her help with the analyses 219 Huntingdon Road many colleagues from the Marine of CITES data, and Sarah Ferriss for Cambridge CB3 0DL, UK Aquarium Council, particularly assisting in assembling information Tel: +44 (0) 1223 277314 Aquilino A. Alvarez, Paul Holthus and and analysing Annex D and GMAD data Fax: +44 (0) 1223 277136 Peter Scott, and all trading companies on Hippocampus spp. We are grateful E-mail: [email protected] who made data available to us for to Neville Ash for reviewing and editing Website: www.unep-wcmc.org inclusion into GMAD. The kind earlier versions of the manuscript. Director: Mark Collins assistance of Akbar, John Brandt, Thanks also for additional John Caldwell, Lucy Conway, Emily comments to Katharina Fabricius, THE UNEP WORLD CONSERVATION Corcoran, Keith Davenport, John Daphné Fautin, Bert Hoeksema, Caroline MONITORING CENTRE is the biodiversity Dawes, MM Faugère et Gavand, Cédric Raymakers and Charles Veron; for assessment and policy implemen- Genevois, Thomas Jung, Peter Karn, providing reprints, to Alan Friedlander, tation arm of the United Nations Firoze Nathani, Manfred Menzel, Julie Hawkins, Sherry Larkin and Tom Environment Programme (UNEP), the Davide di Mohtarami, Edward Molou, Ogawa; and for providing the picture on world’s foremost intergovernmental environmental organization. -

The Red Knob Starfish
Redfish April, 2012 (Issue #10) Specialist Stars the Red Knob Starfish Reef Cichlids Marine Feeding your corals! Pseudotropheus flavus! Surgeons & tangs explored! Marine Aqua One Wavemakers.indd 1 11/04/12 2:55 PM Redfish contents redfishmagazine.com.au 4 About 5 Off the Shelf 7 Pseudotropheus flavus Redfish is: Jessica Drake, Nicole Sawyer, 10 Today in the Fishroom Julian Corlet & David Midgley Email: [email protected] 18 Surgeonfishes Web: redfishmagazine.com.au Facebook: facebook.com/redfishmagazine Twitter: @redfishmagazine 28 Horned Starfish Redfish Publishing. Pty Ltd. PO Box 109 Berowra Heights, 32 Feeding Corals NSW, Australia, 2082. ACN: 151 463 759 38 Community listing This month’s Eye Candy Contents Page Photos cour- tesy: (Top row. Left to Right) ‘Sweetlips’ by Jon Connell ‘Fish pond at the University of Chicago’ by Steve Browne & John Verkleir ‘rainbowfish’ by boscosami@flickr ‘Tarpon’ by Ines Hegedus-Garcia ‘National Arboretum - Koi Pond II’ by Michael Bentley (Bottom row. Left to Right) ‘Being Watched’ by Tony Alter ‘Untitled’ by Keith Bellvay ‘Clownfish’ by Erica Breetoe ‘Yellow Watchman Goby’ by Clay van Schalkwijk Two Caribbean Flamingo Tongue ‘Horned Viper’ by Paul Albertella Snails (Cyphona gibbosum) feeding on a soft-coral (Plexaura flexuosa). Photo by Laszlo Ilyes The Fine Print Redfish Magazine General Advice Warning The advice contained in this publication is general in nature and has been prepared without understanding your personal situ- ation, experience, setup, livestock and/or environmental conditions. This general advice is not a substitute for, or equivalent of, advice from a professional aquarist, aquarium retailer or veterinarian. Distribution We encourage you to share our website address online, or with friends. -

Centropomidae
click for previous page CENTRP 1983 FAO SPECIES IDENTIFICATION SHEETS FISHING AREA 51 (W. Indian Ocean) CENTROPOMIDAE Barramundis, sea perches Body elongate or oblong, compressed, dorsal profile concave at nape. Mouth large, jaws equal or with lower longer than upper; teeth small, in narrow or villiform bands on jaws and on vomer and palatines (roof of mouth), sometimes also on tongue; preopercle with a serrated posterior border or with 2 ridges; opercle with a single spine. Dorsal fin almost wholly separated into 2, with 7 or 8 stronq spines in front, followed by 1 spine and 10 to 15 soft rays; pelvic fins below pectoral fins, with a stronq spine and 5 soft rays; anal fin short, with 3 spines and 8 to 13 soft rays; caudal fin rounded. Scales usually large, ctenoid and adherent; lateral line continued onto caudal fin. Colour: usually dark grey or green above and silvery below. Medium- to large-sized bottom-living fishes occurring in coastal waters, estuaries and lagoons, in depths between about 10 and 30 m. Highly esteemed food and sport fishes taken mainly by artisanal fisheries. dorsal fins almost separate lateral line single spine continued onto tail concave - 2 - FAO Sheets CENTROPOMIDAE Fishing Area 51 SIMILAR FAMILIES OCCURRING IN THE AREA: Serranidae: spinous and soft parts of dorsal fin not as deeply notched; also, colour pattern distinctive and/or caudal fin truncate or weakly emarginate in some. Lethrinidae, Lutjanidae: dorsal fin not deeply notched, head profile not concave over eye and canine teeth present in some. Sciaenidae: lateral line also extends onto tail, but only 2 anal spines. -

Training Manual Series No.15/2018
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CMFRI Digital Repository DBTR-H D Indian Council of Agricultural Research Ministry of Science and Technology Central Marine Fisheries Research Institute Department of Biotechnology CMFRI Training Manual Series No.15/2018 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual This is a limited edition of the CMFRI Training Manual provided to participants of the “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals” organized by the Marine Biotechnology Division of Central Marine Fisheries Research Institute (CMFRI), from 2nd February 2015 - 31st March 2018. Principal Investigator Dr. P. Vijayagopal Compiled & Edited by Dr. P. Vijayagopal Dr. Reynold Peter Assisted by Aditya Prabhakar Swetha Dhamodharan P V ISBN 978-93-82263-24-1 CMFRI Training Manual Series No.15/2018 Published by Dr A Gopalakrishnan Director, Central Marine Fisheries Research Institute (ICAR-CMFRI) Central Marine Fisheries Research Institute PB.No:1603, Ernakulam North P.O, Kochi-682018, India. 2 Foreword Central Marine Fisheries Research Institute (CMFRI), Kochi along with CIFE, Mumbai and CIFA, Bhubaneswar within the Indian Council of Agricultural Research (ICAR) and Department of Biotechnology of Government of India organized a series of training programs entitled “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals”. -

Validation of the Synonymy of the Teleost Blenniid Fish Species
Zootaxa 4072 (2): 171–184 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2016 Magnolia Press ISSN 1175-5334 (online edition) http://doi.org/10.11646/zootaxa.4072.2.2 http://zoobank.org/urn:lsid:zoobank.org:pub:8AC3AF86-10AA-4F57-A86D-3E8BA4F26EE5 Validation of the synonymy of the teleost blenniid fish species Salarias phantasticus Boulenger 1897 and Salarias anomalus Regan 1905 with Ecsenius pulcher (Murray 1887) based on DNA barcoding and morphology GILAN ATTARAN-FARIMANI1, SANAZ ESTEKANI2, VICTOR G. SPRINGER3, OLIVER CRIMMEN4, G. DAVID JOHNSON3 & CAROLE C. BALDWIN3,5 1Chabahar Maritime University, Faculty of Marine Science, Chabahar, Iran 2MSc student, Chabahar Maritime University, Faculty of Marine Science, Chabahar, Iran 3National Museum of Natural History, Smithsonian Institution 4Natural History Museum, London 5Corresponding author. E-mail: [email protected] Abstract As currently recognized, Ecsenius pulcher includes Salarias pulcher (type material has a banded color pattern), S. anom- alus (non-banded), and S. phantasticus (banded). The color patterns are not sex linked, and no other morphological fea- tures apparently distinguish the three nominal species. The recent collection of banded and non-banded specimens of Ecsenius pulcher from Iran has provided the first tissue samples for genetic analyses. Here we review the taxonomic his- tory of E. pulcher and its included synonyms and genetically analyze tissue samples of both color patterns. Salarias anom- alus is retained as a synonym of E. pulcher because DNA barcode data suggest that they represent banded and non-banded color morphs of a single species. Furthermore, the large size of the largest type specimen of S. -
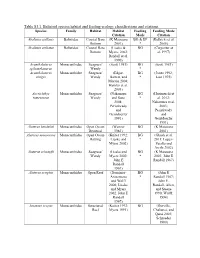
Table S3.1. Balistoid Species Habitat and Feeding Ecology Classifications and Citations
Table S3.1. Balistoid species habitat and feeding ecology classifications and citations. Species Family Habitat Habitat Feeding Feeding Mode Citation Mode Citation Abalistes stellaris Balistidae Coastal Bare (K Matsuura BG & EP (Kulbicki et al. Bottom 2001) * 2005) Abalistes stellatus Balistidae Coastal Bare (Lieske & BG (Carpenter et Bottom Myers, 2002; al. 1997) Randall et al. 1990) Acanthaluteres Monacanthidae Seagrass/ (Scott 1981) BG (Scott 1981) spilomelanurus Weedy * Acanthaluteres Monacanthidae Seagrass/ (Edgar, BG (Jones 1992; vittiger Weedy Barrett, and * Last 1975) Morton 2004; Hyndes et al. 2003) Acreichthys Monacanthidae Seagrass/ (Nakamura BG (Horinouchi et tomentosus Weedy and Sano * al. 2012; 2004; Nakamura et al. Peristiwady 2003; and Peristiwady Geistdoerfer and 1991) Geistdoerfer 1991) Aluterus heudeloti Monacanthidae Open Ocean (Wenner BG (K Matsuura Demersal 1983) 2001) Aluterus monoceros Monacanthidae Open Ocean (Kuiter 1992; BG (Ghosh et al. Rafting Lieske and * 2011; Lopez- Myers 2002) Peralta and Arcila 2002) Aluterus schoepfii Monacanthidae Seagrass/ (Lieske and BG (K Matsuura Weedy Myers 2002; * 2001; John E John E Randall 1967) Randall 1967) Aluterus scriptus Monacanthidae Open Reef (Dominici- BG (John E Arosemena * Randall 1967; and Wolff John E. 2006; Lieske Randall, Allen, and Myers and Steene 2002; John E 1990; Wulff, Randall 1994) 1967) Amanses scopas Monacanthidae Structured (Kuiter 1992; BG (Durville, Reef Myers 1991) Chabanet, and Quod 2003; Schroeder 1980) Balistapus Balistidae Structured (Hiatt and BG & EP (Hiatt and undulatus Reef Strasburg * Strasburg 1960; 1960; Lieske John E. Randall and Myers 1955; John E. 2002; Randall, Allen, McClanahan and Steene and Shafir 1990) 1990; John E. Randall, Allen, and Steene 1990) Balistes capriscus Balistidae Open Ocean (Longley BG & EP (Goldman, Pelagic 1941) * Glasgow, and Falk 2016; Lieske and Myers 2002; Vose and Nelson 1994) Balistes polylepis Balistidae Open Reef (Burgess et BG & EP (Abitia al. -

Annotated Checklist of the Fish Species (Pisces) of La Réunion, Including a Red List of Threatened and Declining Species
Stuttgarter Beiträge zur Naturkunde A, Neue Serie 2: 1–168; Stuttgart, 30.IV.2009. 1 Annotated checklist of the fish species (Pisces) of La Réunion, including a Red List of threatened and declining species RONALD FR ICKE , THIE rr Y MULOCHAU , PA tr ICK DU R VILLE , PASCALE CHABANE T , Emm ANUEL TESSIE R & YVES LE T OU R NEU R Abstract An annotated checklist of the fish species of La Réunion (southwestern Indian Ocean) comprises a total of 984 species in 164 families (including 16 species which are not native). 65 species (plus 16 introduced) occur in fresh- water, with the Gobiidae as the largest freshwater fish family. 165 species (plus 16 introduced) live in transitional waters. In marine habitats, 965 species (plus two introduced) are found, with the Labridae, Serranidae and Gobiidae being the largest families; 56.7 % of these species live in shallow coral reefs, 33.7 % inside the fringing reef, 28.0 % in shallow rocky reefs, 16.8 % on sand bottoms, 14.0 % in deep reefs, 11.9 % on the reef flat, and 11.1 % in estuaries. 63 species are first records for Réunion. Zoogeographically, 65 % of the fish fauna have a widespread Indo-Pacific distribution, while only 2.6 % are Mascarene endemics, and 0.7 % Réunion endemics. The classification of the following species is changed in the present paper: Anguilla labiata (Peters, 1852) [pre- viously A. bengalensis labiata]; Microphis millepunctatus (Kaup, 1856) [previously M. brachyurus millepunctatus]; Epinephelus oceanicus (Lacepède, 1802) [previously E. fasciatus (non Forsskål in Niebuhr, 1775)]; Ostorhinchus fasciatus (White, 1790) [previously Apogon fasciatus]; Mulloidichthys auriflamma (Forsskål in Niebuhr, 1775) [previously Mulloidichthys vanicolensis (non Valenciennes in Cuvier & Valenciennes, 1831)]; Stegastes luteobrun- neus (Smith, 1960) [previously S.