1 Addis Ababa University School of Graduate Studies
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Projet De Motion Sur La Dégradation De
WCC-2012-Res-023-EN Support for national and regional initiatives for the conservation of large mammals in the Sahara RECOGNIZING IUCN’s mission, since its creation, to promote the conservation of biodiversity; AWARE that desert ecosystems and their biodiversity are particularly vulnerable to natural and anthropogenic climate change; RECOGNIZING that the Sahara is very rich in biodiversity, which is often underestimated and is potentially important in the provision of ecosystem services and genetic resources; RECOGNIZING that the populations of large mammals have declined dramatically in desert ecosystems, and in the Sahara in particular; ALARMED that all eight species of Saharan ungulates as well as their subspecies are either threatened with extinction or already extinct, the Bubal Hartebeest (Alcelaphus buselaphus buselaphus) is classified as Extinct, the Scimitar-horned Oryx (Oryx dammah) is Extinct in the Wild and six others are classified as Endangered or Critically Endangered; RECOGNIZING that the African Lion (Panthera leo leo) and the African Wild Dog (Lycaon pictus) have been exterminated from the Sahara and that the Northwest African cheetah (Acinonyx jubatus hecki) is classified as Critically Endangered; AWARE that three of the large mammal species living in the desert require vast ranges in order to survive; NOTING that desert ecosystems have attracted very little interest or support from the global conservation community, despite covering over 17% of the world’s biomass and containing a high level of biodiversity, including -

Confirming the Pleistocene Age of the Qurta Rock
EGYPTIAN ARCHAEOLOGY Confirming the Pleistocene age of the Qurta rock art An article in EA 33 made the case for the existence of Pleistocene rock art at Qurta, c.15km north of Kom Ombo. Further fieldwork and laboratory analyses during 2008-09 have proved its pre- Holocene age as Dirk Huyge and Dimitri A G Vandenberghe discuss. The circumstances of the finding of the Qurta rock art in 2005 were described in our 2008 report in EA 33 (pp.25- 28). At Qurta, on the east bank of the Nile between Edfu and Aswan, three rock art sites have been identified: Qurta I, II and III (henceforth QI, QII and QIII). They are located in higher parts of the Nubian sandstone scarp bordering the Nile floodplain. At each site several rock art locations, panels and individual figures have been identified, with a total of at least 185 distinct images. Naturalistically drawn aurochs (Bos primigenius ) are predominant (over 75% of the total number of drawings), View of Qurta II showing the location of rock art panel QII.4.2, which is partly covered by Nubian sandstone rock debris and sediment accumulations followed by birds, hippopotami, gazelle, fish and hartebeest. In addition, some indeterminate During the 2008 field campaign, it became clear that creatures and several highly-stylized representations of rock art panel QII.4.2 was partly covered by sediment human figures appear at the sites. On the basis of the accumulations that were trapped between the engraved intrinsic characteristics of the rock art, its patination and rock face and coarse Nubian sandstone rock debris degree of weathering, as well as the archaeological and that had become separated from the scarp. -

Gazella Dorcas) in North East Libya
The conservation ecology of the Dorcas gazelle (Gazella dorcas) in North East Libya Walid Algadafi A thesis submitted in partial fulfilment of the requirements of the University of Wolverhampton for the degree of Doctor of Philosophy April 2019 This work and any part thereof has not previously been presented in any form to the University or to any other body whether for the purposes of assessment, publication or any other purpose (unless previously indicated). Save for any express acknowledgements, references and/or bibliographies cited in the work, I confirm that the intellectual content of the work is the result of my own efforts and of no other person. The right of Walid Algadafi to be identified as author of this work is asserted in accordance with ss.77 and 78 of the Copyright, Designs and Patents Act 1988. At this date, copyright is owned by the author. Signature: Date: 27/ 04/ 2019 I ABSTRACT The Dorcas gazelle (Gazella dorcas) is an endangered antelope in North Africa whose range is now restricted to a few small populations in arid, semi-desert conditions. To be effective, conservation efforts require fundamental information about the species, especially its abundance, distribution and genetic factors. Prior to this study, there was a paucity of such data relating to the Dorcas gazelle in Libya and the original contribution of this study is to begin to fill this gap. The aim of this study is to develop strategies for the conservation management of Dorcas gazelle in post-conflict North East Libya. In order to achieve this aim, five objectives relating to current population status, threats to the species, population genetics, conservation and strategic population management were identified. -

African Animals Extinct in the Holocene
SNo Common Name\Scientific Name Extinction Date Range Mammals Prehistoric extinctions (beginning of the Holocene to 1500 AD) Atlas Wild Ass 1 300 North Africa Equus africanus atlanticus Canary Islands Giant Rats 2 Before 1500 AD. Spain (Canary Islands) Canariomys bravoi and Canariomys tamarani Giant Aye-aye 3 1000 AD. Madgascar Daubentonia robusta Giant Fossa 4 Unknown Madgascar Cryptoprocta spelea 5 Hipposideros besaoka 10000 BC. Madgascar Homotherium 6 10000 BC. Africa Homotherium sp. Koala Lemur 7 1420 AD. Madagascar Megaladapis sp. Lava Mouse 8 Before 1500 Spain (Canary Islands) Malpaisomys insularis Malagasy Aardvark 9 200 BC Madagascar Plesiorycteropus sp. Malagasy Hippopotamus 10 1000 AD. Madgascar Hippopotamus sp. Megalotragus 11 10000 BC. Africa Megalotragus sp. North African Elephant 12 300 AD. North Africa Loxodonta africana pharaoensis Pelorovis 13 2000 BC. Africa Pelorovis sp. Sivatherium 14 6000 BC. Africa Sivatherium sp. Recent Extinctions (1500 to present) Atlas Bear 1 1870s North Africa Ursus arctos crowtheri Aurochs Unknown (Africa), 2 North Africa Bos primigenius 1627(Europe) Bluebuck 3 1799 South Africa Hippotragus leucophaeus Bubal Hartebeest 4 1923 North Africa Alcelaphus buselaphus buselaphus Cape Lion 5 1860 South Africa Panthera leo melanochaitus Cape Serval 6 Unknown South Africa Leptailurus serval serval Cape Warthog 7 1900 South Africa Phacochoerus aethiopicus aethiopicus Kenya Oribi 8 Unknown Kenya Ourebia ourebi kenyae Large Sloth Lemur 9 1500s Madagascar Palaeopropithecus sp. Lesser Mascarene Flying Fox 10 -

The Gazelle in Ancient Egyptian Art Image and Meaning
Uppsala Studies in Egyptology - 6 - Department of Archaeology and Ancient History Uppsala University For my parents Dorrit and Hindrik Åsa Strandberg The Gazelle in Ancient Egyptian Art Image and Meaning Uppsala 2009 Dissertation presented at Uppsala University to be publicly examined in the Auditorium Minus of the Museum Gustavianum, Uppsala, Friday, October 2, 2009 at 09:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Abstract Strandberg, Åsa. 2009. The Gazelle in Ancient Egyptian Art. Image and Meaning. Uppsala Studies in Egyptology 6. 262 pages, 83 figures. Published by the Department of Archaeology and Ancient History, Uppsala University. xviii +262 pp. ISSN 1650-9838, ISBN 978-91-506-2091-7. This thesis establishes the basic images of the gazelle in ancient Egyptian art and their meaning. A chronological overview of the categories of material featuring gazelle images is presented as a background to an interpretation. An introduction and review of the characteristics of the gazelle in the wild are presented in Chapters 1-2. The images of gazelle in the Predynastic material are reviewed in Chapter 3, identifying the desert hunt as the main setting for gazelle imagery. Chapter 4 reviews the images of the gazelle in the desert hunt scenes from tombs and temples. The majority of the motifs characteristic for the gazelle are found in this context. Chapter 5 gives a typological analysis of the images of the gazelle from offering processions scenes. In this material the image of the nursing gazelle is given particular importance. Similar images are also found on objects, where symbolic connotations can be discerned (Chapter 6). -

Gazella Dorcas and Gazella Leptoceros) in Egypt
Contemporary status and distribution of gazelle species (Gazella dorcas and Gazella leptoceros) in Egypt by Husam El Alqamy and Sherif Baha El Din Abstract. Only two gazelle species are currently present in a wild state in Egypt. These are Dor- cas Gazelle (Gazella dorcas) and Slender-horned Gazelle (Gazella leptoceros). The latest infor- mation available about the status and distribution of these two species collected during the period 1997–2005 indicates that the population size and range of both species continue to shrink at dif- ferent rates. The conservation status of the two species is reviewed and a quantitative estimation for the range of the two species is provided using IUCN’s Area of Occurrence and Area of Occu- pancy guidelines. Kurzfassung. Gegenwärtig kommen in Ägypten in freier Wildbahn nur noch zwei Gazellenarten vor: die Dorkasgazelle (Gazella dorcas) und die Dünengazelle (Gazella leptoceros). Neue Infor- mationen aus den Jahren 1997–2005 über den Status und die Verbreitung zeigen, dass sowohl die Populationen beider Arten als auch die Größe ihres Verbreitungsgebietes in unterschiedlicher Ge- schwindigkeit weiter abnehmen. Der Schutzstatus beider Arten wird dargestellt und eine quantita- tive Analyse des Vorkommensgebietes anhand der von IUCN entwickelten Richtlinien „Area of Occurrence“ und „Area of Occupancy“ wird vorgenommen. Keywords. Antelopes, gazelles, range, conservation status, distribution, Area of Occurrence, Area of Occupancy. Introduction Except for the Mediterranean littoral belt and a few mountain peaks, the Egyptian deserts are classified as hyper-arid deserts receiving less than 100 mm of annual precipitation (AYAD & GHABOUR 1986). These deserts are therefore of very low productivity and many animal populations, especially large mammals, have to cover large distances in the pursuit of fa- vourable habitats and feeding grounds. -

25 Years On: a Look at Endangered Species
25 Years On: a Look at Endangered Species Richard Fitter What has happened to the animals and birds listed as endangered species 25 years ago at IUCN's first technical meeting? The FPS Hon. Secretary investigates their present status and looks at the conservation methods that have proved most effective. In August 1949 two lists of endangered species were drawn up, one of fourteen mammals, the other of thirteen birds, all described as 'in need of emergency action if they are to be saved from extinction'. This was at the First Technical Conference, at Lake Success, of the International Union for the Protection of Nature, as IUCN (the International Union for Conservation of Nature and Natural Resources) was then called. It is of some interest now, 25 years later, to review these lists and see what, if anything has been done to save these animals. The mammals comprised two marsupials—thylacine and western banded anteater—chinchilla, Asiatic lion, Caribbean and Mediterranean monk seals, Addo bush elephant, mountain zebra, great Indian and Javan rhinos, Burmese brow-antlered deer, and three bovids—European bison, North African hartebeest and giant sable antelope. Of these fourteen, two are now regarded as extinct: the Caribbean monk seal Monachus tropicalis, last definitely reported in 1952, which a very recent and thorough survey has failed to rediscover; and the North African or bubal hartebeest Alcelaphus buselaphus buselaphus, which has not been reliably reported since 1925. Of the thylacine Thylacinus cynocephalus of Tasmania there has also been no recent scientifically verified sighting, though there have been a number of apparently well-founded but still unverified ones. -

Alcelaphus Buselaphus Caama – Red Hartebeest
Alcelaphus buselaphus caama – Red Hartebeest Taxonomic status: Subspecies Taxonomic notes: Following Gosling and Capellini (2013), and in contrast to Grubb (2005), this species is here considered to include both Red Hartebeest A. caama and Lichtenstein's Hartebeest A. lichtensteinii. A total of eight subspecies are recognized, of which Red Hartebeest occurs in the assessment region and possibly (see discussion below) Lichtenstein's Hartebeest. The eighth and nominate subspecies, the Bubal Hartebeest A. b. buselaphus, from North Africa is now Extinct (IUCN SSC Antelope Specialist Group 2016). Assessment Rationale Beryl Wilson The Red Hartebeest, although historically reduced from overhunting, is now common within the assessment Regional Red List status (2016) region, having been reintroduced into a number of formal Alcelaphus b. caama Least Concern and private protected areas across its range. The wildlife ranching industry also harbours a large number of animals Alcelaphus b. lichtensteinii Not Evaluated* on privately owned game farms and reserves. The National Red List status (2004) population is widespread on formally protected areas and private land and has increased significantly on formally Alcelaphus b. caama Least Concern protected areas at least over the past three generations Alcelaphus b. lichtensteinii Not Evaluated (1992–2015). Globally, Red Hartebeest is the most numerous subspecies (c. 130,000 animals) and is Reasons for change No change increasing. Within the assessment region, there are at least 14,849 mature animals (assuming a 70% mature Global Red List status (2016) population structure) on formally protected areas (with Alcelaphus b. caama Least Concern three subpopulations > 1,000 mature individuals in Kgalagadi Transfrontier Park, Golden Gate Highlands Alcelaphus b. -
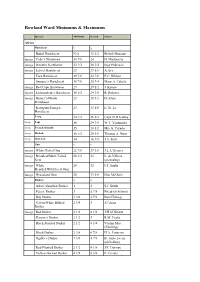
Rowland Ward Minimums & Maximums
Rowland Ward Minimums & Maximums Species Minimum Record Owner Africa Hartebeest x x x Bubal Hartebeest N/A 15 1/2 British Museum image Coke’s Hartebeest 18 7/8 24 D. Mackenzie image Western Hartebeest 22 1/2 28 3/4 Inge Pederson image Lelwel Hartebeest 23 27 5/8 A.Avy Tora Hartebeest 19 7/8 22 7/8 F.C. Hibben Swayne’s Hartebeest 16 7/8 20 3/4 Mary A. Cabela image Red/Cape Hartebeest 23 29 1/2 J. Krause image Lichtenstein’s Hartebeest 18 1/2 24 3/8 R. Rohwer image Hunter’s/Hirola 23 28 1/2 H. Klein Hartebeest Korrigum/Senegal 23 33 1/8 L. St. Lo Hartebeest Tiang 20 1/2 26 1/2 Capt. D.H Gawne image Topi 16 24 3/8 W.T. Yoshimoto image Tsessebe/Sassaby 15 18 1/2 Mrs A. Curado image Blesbok 16 1/2 20 5/8 Thomas A. Hunt image Bontebok 14 16 3/8 J.A. Feist Gnu x x x image White-Tailed Gnu 22 7/8 29 3/8 J.L. L’Ecuyer image Brindled/Black-Tailed 28 1/2 36 L. de Villiers Gnu (picked up) image White 28 32 I.T. Smith Bearded/Wildebeest Gnu image Nyasaland Gnu 28 33 1/8 Don McGuirt Duikers x x x Aders’/Zanzibar Duiker 1 2 S.J. Smith Peters’ Duiker 3 5 7/8 Friedrich Schmitt Bay Duiker 2 3/8 4 7/8 Bela Hidvegi Gabon/White Bellied 2 3/4 5 J.Cousin Duiker image Red Duiker 2 1/2 4 1/8 J.H.M Niblett Harvey’s Duiker 2 1/2 5 R.M. -

Lost Animals: Extinction and the Photographic Record Free
FREE LOST ANIMALS: EXTINCTION AND THE PHOTOGRAPHIC RECORD PDF Errol Fuller | 256 pages | 21 Nov 2013 | Bloomsbury Publishing PLC | 9781408172155 | English | London, United Kingdom Top 10 Extinct Animals | HowStuffWorks The movies may have filled your mind with visions of wooly mammoths and velociraptors walking the Earth once more, but de-extinction—that is, bringing extinct species back to life—is probably going to be a lot more boring Lost Animals: Extinction and the Photographic Record that. Now that the science is seemingly within reach, researchers at the University of California, Santa Barbra have Lost Animals: Extinction and the Photographic Record up some guidelines for responsibly bringing back a species. Sorry, mastodon fans: They're heavy on responsibility and light on charismatic megafauna. First of all, UCSB ecologist Douglas McCauley and his colleagues say, the ideal first candidates for this would be recently extinct animals. Think less stegosaurus, more Christmas Island pipistrelle batwhich was deemed extinct in Long-dead species could be unprepared for the modern ecosystem. The pipistrelle bat could fit right in on day one. Secondly, they say, animals should be chosen based on what they contribute to their ecosystems. The pipistrelle bat is a good example because it was an insect-eater in its environment and could easily enter that role again. A mammoth would be an invasive species, and their introduction might follow a track that's more like what happened when infamous drug lord Pablo Escobar brought hippos to Columbia. While the hippos seem to enjoy themselves, the rest of the ecosystem did not. The third rule is that the animal must be brought back to meaningful abundance levels. -

The Status and Distribution of Mediterranean Mammals
THE STATUS AND DISTRIBUTION OF MEDITERRANEAN MAMMALS Compiled by Helen J. Temple and Annabelle Cuttelod AN E AN R R E IT MED The IUCN Red List of Threatened Species™ – Regional Assessment THE STATUS AND DISTRIBUTION OF MEDITERRANEAN MAMMALS Compiled by Helen J. Temple and Annabelle Cuttelod The IUCN Red List of Threatened Species™ – Regional Assessment The designation of geographical entities in this book, and the presentation of material, do not imply the expression of any opinion whatsoever on the part of IUCN or other participating organizations, concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland and Cambridge, UK Copyright: © 2009 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Red List logo: © 2008 Citation: Temple, H.J. and Cuttelod, A. (Compilers). 2009. The Status and Distribution of Mediterranean Mammals. Reprint, Gland, Switzerland and Malaga, Spain : IUCN, 2009. vii+32pp. ISBN: 978-2-8317-1163-8 Legal Deposit: Cover design: Cambridge Publishers Cover photo: Iberian lynx Lynx pardinus © Antonio Rivas/P. Ex-situ Lince Ibérico All photographs used in this publication remain the property of the original copyright holder (see individual captions for details). -

African Safari (4-6) Extensions
Frisch’s Outreach: African Safari (4-6) Extensions At a glance This program will allow your students to learn about the Geography and Climate of Africa. And where these animals live.. Goal Continent and learn where these This class is designed to familiarize habitats are located. students with Africa. The students will The students will understand how learn what its climate is like, what types the program animal lives and of animals live there and how they survives in its habitat. survive. The student will be able to identify what is the range of the Objectives program animal. The students will gain an understanding of the basic Theme features of maps and how to read Africa has a unique geography that has them. an impact on its wildlife. The students will know where the major geographic features of Sub-themes Africa are located. What types of habitats are found The students will be able to in Africa. identify what types of habitats What adaptations do animals are found on the African have to survive in their habitats. Academic standards Ohio Science Academic Grade 5 Life Sciences Content Standards Benchmark C – 4,5,6, Grade 7 Earth and Space Sciences Benchmark C-8, Grade 10 Life Sciences Benchmark E-14, Benchmark F-14,16,17, Benchmark G-18,19, Title (X-X): Extensions Page 1 of 8 Grade 11 Earth and Space Sciences Benchmark B-4, Benchmark C-12, Life Sciences Benchmark E-6,8, Grade 12 Earth and Space Sciences Benchmark B-5, Life Sciences Benchmark C-12 Benchmark E-7,8, Kentucky Core Content— Life Science: Science Grade 4 The Characteristics