Comparing Hypotheses Proposed by Two Conceptual Models for Stream
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

How to Determine Whether a Watercourse Is a River, Ephemeral
Date: 29th August 2019 To: Environmental Policy and Environmental Regulation Greater Wellington Regional Council From: Michael Greer Aquanet Consulting Ltd How to determine whether a watercourse is a river, ephemeral flow path, highly modified river or stream or artificial watercourse: A guidance note for Greater Wellington Regional Council officers 1 Introduction The provisions of the Resource Management Act 1991 (RMA) and the proposed Natural Resources Plan (pNRP) mean that certain activities are subject to different restrictions when undertaken on the bed of different types of watercourses. When determining how the pNRP policies and rules apply to a given watercourse, it must first be determined if the watercourse meets the RMA definition of a river, or if it is an artificial watercourse and therefore not covered by Section 13 of the RMA. If a watercourse is found to be a river, it also needs to be determined whether: It is an ephemeral flow path under the pNRP (as ephemeral flow paths are excluded from the definition of a surface water body and therefore certain rules do not apply); or It is a highly modified river or stream under the pNRP (only relevant when determining whether a watercourse can be managed under Rule R121). The purpose of this document is to describe a methodology that can be used by Greater Wellington Regional Council (GWRC) officers and consent applicants to determine whether a watercourse is: A river, and therefore subject to relevant rules in the pNRP; A highly modified river or stream for the purpose of Rule R121 only; An ephemeral flow path that is not covered by Rules R42, R48, R59 and R60 and is exempt from the directive in Policy P102 to avoid reclamation; or Artificial and not subject to the rules in Section 5.5 of the pNRP. -

Watercourse Centerline and Habitat Feature Mapping
Watercourse Centerline and Habitat Feature Mapping 3.1 Purpose The purpose of this SHIM module is to present the methods for mapping watercourse locations and describing watercourse attributes and habitats. These methods are designed for use in conjunction with SHIM Module 4 (Riparian Area Classification and Stream Channel Cross-Sections), and SHIM Module 5 (GPS Surveying Procedures) and describe field and attribute in the “data dictionary” of the Global Positioning System used in SHIM watercourse field surveying. Accurate spatial mapping of watercourses is critical to developing precise land use plans and Official Community Plans (OCP) in urban and many rural areas. Streams, wetlands and watercourses can be easily damaged or destroyed by land use development. Knowledge of watercourse extent and location is critical for providing information to help guide development and protect watercourses. The history of human development has shown that sensitive watercourses need protection and their precise location should be clearly understood and incorporated into local planning and development maps. It is not unusual for watercourses to be incorrectly mapped or even omitted entirely from municipal and provincial plans and maps (Fig. 3.1, see Fig. 2.1). It can be expensive to use land surveying techniques to map streams and watercourse to levels accurate and reliable enough to use in community and municipal land use plans. Recent developments in Global Positioning System (GPS) differential technology (SHIM Module 5) have enabled the quick, accurate, and inexpensive survey techniques to inventory and map resources and watercourses. The objective of Module 3 is to describe the SHIM standard for watercourse mapping using a resource survey grade differential GPS unit (here a Trimble Pathfinder, Appendix A). -

Protecting Wildlife for the Future
CINDERELLA SCORES A HAT-TRICK! 3 years, 3 projects, 3km of winterbourne chalkstream restored Introduction The Dorset Wild Rivers project and the Monitoring & Results Environment Agency have created three successful winterbourne restoration projects In order to measure the impacts of that have delivered a number of outcomes our work, pre and post work including Biodiversity Action Plan (BAP) macroinvertebrate and fish targets, working towards Good Ecological monitoring is being carried out as Status (GES under the Water Framework part of the project. Directive (WFD) and building resilience to climate change. A more diverse habitat supporting diverse wildlife has been created. Winterbournes are rare chalk streams which The bankside vegetation has been are groundwater fed and only flow at certain manipulated to provide a mixture of times of the year as groundwater levels in the both shaded and more open sections aquifer fluctuate. They support a range of of channel and a more species rich specialist wildlife adapted to this unusual margin. flow regime, including a number of rare or scarce invertebrates. Our macro invertebrate sampling indicates that the work has been a So called “Cinderella” chalkstreams because great success: the rare mayfly larva they are so often overlooked. Their Paraleptophlebia werneri (Red Data ecological value is often degraded as a result Book 3), and the notable blackfly of pressures from agricultural practices, land larva Metacnephia amphora, were drainage, urban and infrastructure found in the stream only 6 months development, abstraction and flood after the work was completed. defences. The Conservation value of the new Over centuries, the spring-fed South channel was reassessed using the Winterbourne in Dorset has been degraded. -

Stream Restoration, a Natural Channel Design
Stream Restoration Prep8AICI by the North Carolina Stream Restonltlon Institute and North Carolina Sea Grant INC STATE UNIVERSITY I North Carolina State University and North Carolina A&T State University commit themselves to positive action to secure equal opportunity regardless of race, color, creed, national origin, religion, sex, age or disability. In addition, the two Universities welcome all persons without regard to sexual orientation. Contents Introduction to Fluvial Processes 1 Stream Assessment and Survey Procedures 2 Rosgen Stream-Classification Systems/ Channel Assessment and Validation Procedures 3 Bankfull Verification and Gage Station Analyses 4 Priority Options for Restoring Incised Streams 5 Reference Reach Survey 6 Design Procedures 7 Structures 8 Vegetation Stabilization and Riparian-Buffer Re-establishment 9 Erosion and Sediment-Control Plan 10 Flood Studies 11 Restoration Evaluation and Monitoring 12 References and Resources 13 Appendices Preface Streams and rivers serve many purposes, including water supply, The authors would like to thank the following people for reviewing wildlife habitat, energy generation, transportation and recreation. the document: A stream is a dynamic, complex system that includes not only Micky Clemmons the active channel but also the floodplain and the vegetation Rockie English, Ph.D. along its edges. A natural stream system remains stable while Chris Estes transporting a wide range of flows and sediment produced in its Angela Jessup, P.E. watershed, maintaining a state of "dynamic equilibrium." When Joseph Mickey changes to the channel, floodplain, vegetation, flow or sediment David Penrose supply significantly affect this equilibrium, the stream may Todd St. John become unstable and start adjusting toward a new equilibrium state. -

Historic Erie Canal Aqueduct & Broad Street Corridor
HISTORIC ERIE CANAL AQUEDUCT & BROAD STREET CORRIDOR MASTER PLAN MAY 2009 PREPARED FOR THE CITY OF ROCHESTER Copyright May 2009 Cooper Carry All rights reserved. Design: Cooper Carry 2 Historic Erie Canal AQUedUct & Broad Street Corridor Master Plan HISTORIC ERIE CANAL AQUEDUCT & BROAD STREET CORRIDOR 1.0 MASTER PLAN TABLE OF CONTENTS 5 1.1 EXECUTIVE SUMMARY 23 1.2 INTRODUCTION 27 1.3 PARTICIPANTS 33 2.1 SITE ANALYSIS/ RESEARCH 53 2.2 DESIGN PROCESS 57 2.3 HISTORIC PRECEDENT 59 2.4 MARKET CONDITIONS 67 2.5 DESIGN ALTERNATIVES 75 2.6 RECOMMENDATIONS 93 2.7 PHASING 101 2.8 INFRASTRUCTURE & UTILITIES 113 3.1 RESOURCES 115 3.2 ACKNOWLEDGEMENTS Historic Erie Canal AQUedUct & Broad Street Corridor Master Plan 3 A city... is the pulsating product of the human hand and mind, reflecting man’s history, his struggle for freedom, creativity and genius. - Charles Abrams VISION STATEMENT: “Celebrating the Genesee River and Erie Canal, create a vibrant, walkable mixed-use neighborhood as an international destination grounded in Rochester history connecting to greater city assets and neighborhoods and promoting flexible mass transit alternatives.” 4 Historic Erie Canal AQUedUct & Broad Street Corridor Master Plan 1.1 EXECUTIVE SUMMARY CREATING A NEW CANAL DISTRICT Recognizing the unrealized potential of the area, the City of the historic experience with open space and streetscape initiatives Rochester undertook a planning process to develop a master plan which coordinate with the milestones of the trail. for the Historic Erie Canal Aqueduct and adjoining Broad Street Corridor. The resulting Master Plan for the Historic Erie Canal Following the pathway of the original canal, this linear water Aqueduct and Broad Street Corridor represents a strategic new amenity creates a signature urban place drawing visitors, residents, beginning for this underutilized quarter of downtown Rochester. -

River Incision Into Bedrock: Mechanics and Relative Efficacy of Plucking, Abrasion and Cavitation
River incision into bedrock: Mechanics and relative efficacy of plucking, abrasion and cavitation Kelin X. Whipple* Department of Earth, Atmospheric, and Planetary Science, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139 Gregory S. Hancock Department of Geology, College of William and Mary, Williamsburg, Virginia 23187 Robert S. Anderson Department of Earth Sciences, University of California, Santa Cruz, California 95064 ABSTRACT long term (Howard et al., 1994). These conditions are commonly met in Improved formulation of bedrock erosion laws requires knowledge mountainous and tectonically active landscapes, and bedrock channels are of the actual processes operative at the bed. We present qualitative field known to dominate steeplands drainage networks (e.g., Wohl, 1993; Mont- evidence from a wide range of settings that the relative efficacy of the gomery et al., 1996; Hovius et al., 1997). As the three-dimensional structure various processes of fluvial erosion (e.g., plucking, abrasion, cavitation, of drainage networks sets much of the form of terrestrial landscapes, it is solution) is a strong function of substrate lithology, and that joint spac- clear that a deep appreciation of mountainous landscapes requires knowl- ing, fractures, and bedding planes exert the most direct control. The edge of the controls on bedrock channel morphology. Moreover, bedrock relative importance of the various processes and the nature of the in- channels play a critical role in the dynamic evolution of mountainous land- terplay between them are inferred from detailed observations of the scapes (Anderson, 1994; Anderson et al., 1994; Howard et al., 1994; Tucker morphology of erosional forms on channel bed and banks, and their and Slingerland, 1996; Sklar and Dietrich, 1998; Whipple and Tucker, spatial distributions. -

The Natural Capital of Temporary Rivers: Characterising the Value of Dynamic Aquatic–Terrestrial Habitats
VNP12 The Natural Capital of Temporary Rivers: Characterising the value of dynamic aquatic–terrestrial habitats. Valuing Nature | Natural Capital Synthesis Report Lead author: Rachel Stubbington Contributing authors: Judy England, Mike Acreman, Paul J. Wood, Chris Westwood, Phil Boon, Chris Mainstone, Craig Macadam, Adam Bates, Andy House, Dídac Jorda-Capdevila http://valuing-nature.net/TemporaryRiverNC Suggested citation: Stubbington, R., England, J., Acreman, M., Wood, P.J., Westwood, C., Boon, P., The Natural Capital of Mainstone, C., Macadam, C., Bates, A., House, A, Didac, J. (2018) The Natural Capital of Temporary Temporary Rivers: Rivers: Characterising the value of dynamic aquatic- terrestrial habitats. Valuing Nature Natural Capital Characterising the value of dynamic Synthesis Report VNP12. The text is available under the Creative Commons aquatic–terrestrial habitats. Attribution-ShareAlike 4.0 International License (CC BY-SA 4.0) Valuing Nature | Natural Capital Synthesis Report Contents Introduction: Services provided by wet and the natural capital of temporary rivers.............. 4 dry-phase assets in temporary rivers................33 What are temporary rivers?...................................... 4 The evidence that temporary rivers deliver … services during dry phases...................34 Temporary rivers in the UK..................................... 4 Provisioning services...................................34 The natural capital approach Regulating services.......................................35 to ecosystem protection............................................ -

STREAMS and RUNNING WATER Hydrologic Cycle •What Happens to Precipitation STREAM •River, Creek, Etc
STREAMS AND RUNNING WATER Hydrologic Cycle •What happens to precipitation STREAM •River, Creek, etc. • Banks, Bed • Confined to a channel –Except in time of flood •Floodplain •Longtitudinal Profile Headwaters, mouth Cross-profile V-shaped SHEETWASH –Unchanneled, thin sheet of flowing water – Results in Sheet Erosion Drainage Basin •Tributary • Divide • Examples: –Mississippi River – Amazon River Drainage Patterns •As seen from above • Dendritic (most common) • Radial • Trellis- in tilted or folded layered rock Factors affecting stream erosion and deposition •Velocity- –Distribution in cross-section •Gradient –Usually decreases downstream – Effect on deposition and erosion •Channel shape and roughness • Discharge- –Volume of water moving past a place in time •Usually given as cubic feet per second (cfs) –Usually increases downstream •Discharge- –Volume of water moving past a place in time •Usually given as cubic feet per second (cfs) –Usually increases downstream American River Discharge •In Sacramento: • Average 3,741 c.f.s. • Usual Range: –Winter 9,000 cfs – Last drought 250 cfs •1986 flood 130,000 cfs Stream Erosion •Hydraulic action • Solution- Dissolves rock • Abrasion (by sand and gravel) –Potholes •Deepens valley by eroding river bed • Lateral erosion of banks on outside of curves Transportation of Sediment •Bed load • Suspended load • Dissolved load Deposition •Bars- –moved in floods – deposited as water slows – Placer Deposits (gold, etc.) – Braided Streams •heavy load of sand and gravel • commonly from glacier outwash streams -

The Nelson Ranch Located Along the Shasta River Has Two Flow Gaging
Baseline Assessment of Salmonid Habitat and Aquatic Ecology of the Nelson Ranch, Shasta River, California Water Year 2007 Jeffrey Mount, Peter Moyle, and Michael Deas, Principal Investigators Report prepared by: Carson Jeffres (Project lead), Evan Buckland, Bruce Hammock, Joseph Kiernan, Aaron King, Nickilou Krigbaum, Andrew Nichols, Sarah Null Report prepared for: United States Bureau of Reclamation Klamath Area Office Center for Watershed Sciences University of California, Davis • One Shields Avenue • Davis, CA 95616-8527 Table of Contents 1. EXECUTIVE SUMMARY..................................................................................................................................2 2. INTRODUCTION...............................................................................................................................................6 3. ACKNOWLEDGEMENTS .................................................................................................................................6 4. SITE DESCRIPTION.........................................................................................................................................7 5. HYDROLOGY.....................................................................................................................................................8 5.1. STAGE-DISCHARGE RATING CURVES .......................................................................................................9 5.2. PRECIPITATION........................................................................................................................................11 -
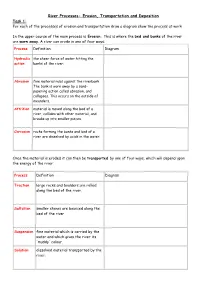
River Processes- Erosion, Transportation and Deposition Task 1: for Each of the Processes of Erosion and Transportation Draw a Diagram Show the Process at Work
River Processes- Erosion, Transportation and Deposition Task 1: For each of the processes of erosion and transportation draw a diagram show the process at work In the upper course of the main process is Erosion. This is where the bed and banks of the river are worn away. A river can erode in one of four ways: Process Definition Diagram Hydraulic the sheer force of water hitting the action banks of the river: Abrasion fine material rubs against the riverbank The bank is worn away by a sand- papering action called abrasion, and collapses. This occurs on the outside of meanders. Attrition material is moved along the bed of a river, collides with other material, and breaks up into smaller pieces. Corrosion rocks forming the banks and bed of a river are dissolved by acids in the water. Once the material is eroded it can then be transported by one of four ways, which will depend upon the energy of the river: Process Definition Diagram Traction large rocks and boulders are rolled along the bed of the river. Saltation smaller stones are bounced along the bed of the river Suspension fine material which is carried by the water and which gives the river its 'muddy' colour. Solution dissolved material transported by the river. In the middle and lower course, the land is much flatter, this means that the river is flowing more slowly and has much less energy. The river starts to deposit (drop) the material that it has been carry Deposition Challenge: Add labels onto the diagram to show where all of the processes could be happening in the river channel. -

Experiments on Patterns of Alluvial Cover and Bedrock Erosion in a Meandering Channel Roberto Fernández1, Gary Parker1,2, Colin P
Earth Surf. Dynam. Discuss., https://doi.org/10.5194/esurf-2019-8 Manuscript under review for journal Earth Surf. Dynam. Discussion started: 27 February 2019 c Author(s) 2019. CC BY 4.0 License. Experiments on patterns of alluvial cover and bedrock erosion in a meandering channel Roberto Fernández1, Gary Parker1,2, Colin P. Stark3 1Ven Te Chow Hydrosystems Laboratory, Department of Civil and Environmental Engineering, University of Illinois at 5 Urbana-Champaign, Urbana, IL 61801, USA 2Department of Geology, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA 3Lamont Doherty Earth Observatory, Columbia University, Palisades, NY 10964, USA Correspondence to: Roberto Fernández ([email protected]) Abstract. 10 In bedrock rivers, erosion by abrasion is driven by sediment particles that strike bare bedrock while traveling downstream with the flow. If the sediment particles settle and form an alluvial cover, this mode of erosion is impeded by the protection offered by the grains themselves. Channel erosion by abrasion is therefore related to the amount and pattern of alluvial cover, which are functions of sediment load and hydraulic conditions, and which in turn are functions of channel geometry, slope and sinuosity. This study presents the results of alluvial cover experiments conducted in a meandering channel flume of high fixed 15 sinuosity. Maps of quasi-instantaneous alluvial cover were generated from time-lapse imaging of flows under a range of below- capacity bedload conditions. These maps were used to infer patterns -

COLD-BASED GLACIERS in the WESTERN DRY VALLEYS of ANTARCTICA: TERRESTRIAL LANDFORMS and MARTIAN ANALOGS: David R
Lunar and Planetary Science XXXIV (2003) 1245.pdf COLD-BASED GLACIERS IN THE WESTERN DRY VALLEYS OF ANTARCTICA: TERRESTRIAL LANDFORMS AND MARTIAN ANALOGS: David R. Marchant1 and James W. Head2, 1Department of Earth Sciences, Boston University, Boston, MA 02215 [email protected], 2Department of Geological Sciences, Brown University, Providence, RI 02912 Introduction: Basal-ice and surface-ice temperatures are contacts and undisturbed underlying strata are hallmarks of cold- key parameters governing the style of glacial erosion and based glacier deposits [11]. deposition. Temperate glaciers contain basal ice at the pressure- Drop moraines: The term drop moraine is used here to melting point (wet-based) and commonly exhibit extensive areas describe debris ridges that form as supra- and englacial particles of surface melting. Such conditions foster basal plucking and are dropped passively at margins of cold-based glaciers (Fig. 1a abrasion, as well as deposition of thick matrix-supported drift and 1b). Commonly clast supported, the debris is angular and sheets, moraines, and glacio-fluvial outwash. Polar glaciers devoid of fine-grained sediment associated with glacial abrasion include those in which the basal ice remains below the pressure- [10, 12]. In the Dry Valleys, such moraines may be cored by melting point (cold-based) and, in extreme cases like those in glacier ice, owing to the insulating effect of the debris on the the western Dry Valleys region of Antarctica, lack surface underlying glacier. Where cored by ice, moraine crests can melting zones. These conditions inhibit significant glacial exceed the angle of repose. In plan view, drop moraines closely erosion and deposition.