Tor De Ciências Biológicas
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Jaguars As Landscape Detectives for the Conservation of Atlantic Forests in Brazil
Jaguars as Landscape Detectives for the Conservation of Atlantic Forests in Brazil by Laury Cullen Jr. Thesis submitted for the degree of Doctor of Philosophy Biodiversity Management Durrell Institute of Conservation and Ecology (DICE) University of Kent Canterbury November 2006 ii Dedication In dedication to the loving memory of my father Laury Cullen (1934-2002) (Photo by Laury Cullen Jr.) “ the jaguar was sent to the world as a test of the will and integrity of first humans” (Colombian indigenous myth, Davis 1996) iii Abstract In this thesis, I show how the jaguar Panthera onca can be used as a landscape detective. A landscape detective is defined as a species that helps determine how to manage landscapes and to design and manage protected area networks. Life history and behavioural features of jaguar make them potentially suitable as landscape species. The main aim of this study is to use the jaguar as a landscape detective to develop a network of core protected areas for the Upper Paraná Region, which lies in the highly threatened Atlantic Forest of Brazil. Information was collected on jaguar density and home range, which was combined with habitat requirements of the species and GIS-generated maps of land cover to develop a map of habitat suitability. This map was used to understand the spatial structure of the jaguar metapopulation, identifying habitat patches of high suitability where jaguar populations exist and are likely to survive over the long-term. Camera trapping and capture-recapture models were used to derive jaguar population size in Morro do Diabo State Park (MDSP). -

Texto Completo.Pdf
ÉRICA MANGARAVITE ESTRUTURA E DIVERSIDADE GENÉTICA NO COMPLEXO Cedrela fissilis (MELIACEAE) ESTIMADAS COM MARCADORES MICROSSATÉLITES Dissertação apresentada à Universidade Federal de Viçosa, como parte das exigências do Programa de Pós- Graduação em Genética e Melhoramento, para a obtenção do título de Magister Scientiae. VIÇOSA MINAS GERAIS - BRASIL 2012 i Ficha catalográfica preparada pela Seção de Catalogação e Classificação da Biblioteca Central da UFV T Mangaravite, Érica, 1987- M277e Estrutura e diversidade genética no complexo 2012 Cedrela fissilis (Meliaceae) estimadas com marcadores microssatélites / Érica Mangaravite. – Viçosa, MG, 2012. viii, 32f. : il. ; (algumas col.) ; 29cm. Orientador: Luiz Orlando de Oliveira. Dissertação (mestrado) - Universidade Federal de Viçosa. Referências bibliográficas: f. 26-32 1. Cedrela fissilis - Genética. 2. Cedrela fissilis - Distribuição geográfica. 3. Filogeografia. 4. Genética florestal. 5. Diversidade de plantas - Conservação. 6. Genética de populações. 7. Microssatélites (Genética). I. Universidade Federal de Viçosa. Departamento de Biologia Geral. Programa de Pós-Graduação em Genética e Melhoramento. II. Título. CDD 22. ed. 583.77 ÉRICA MANGARAVITE ESTRUTURA E DIVERSIDADE GENÉTICA NO COMPLEXO Cedrela fissilis (MELIACEAE) ESTIMADAS COM MARCADORES MICROSSATÉLITES Dissertação apresentada à Universidade Federal de Viçosa, como parte das exigências do Programa de Pós- Graduação em Genética e Melhoramento para a obtenção do título de Magister Scientiae Aprovada em 5 de Setembro de 2012. _________________________________ _________________________________ João Augusto Alves Meira Neto Christina Cleo Vinson (Coorientadora) __________________________________________ Luiz Orlando de Oliveira (Orientador) i Aos meus pais, José Carlos e Maria Julia, Ao namorado, Diego Volpini, Aos irmãos, Igor e Vítor, Dedico! ii AGRADECIMENTOS À Universidade Federal de Viçosa (UFV), pela oportunidade de realizar o mestrado em Genética e Melhoramento e de cumprir este trabalho. -

Hemiptera: Miridae)
LÍVIA AGUIAR COELHO CONTRIBUIÇÃO À TAXONOMIA E BIOGEOGRAFIA DO GÊNERO Prepops REUTER, 1905 (HEMIPTERA: MIRIDAE) Tese apresentada à Universidade Federal de Viçosa, como parte das exigências do Programa de Pós-Graduação em Entomologia, para obtenção do título de Doctor Scientiae. VIÇOSA MINAS GERAIS - BRASIL 2012 Ficha catalográfica preparada pela Seção de Catalogação e Classificação da Biblioteca Central da UFV T Coelho, Lívia Aguiar, 1981- C67c Contribuição à taxonomia e biogeografia do gênero Prepops 2012 Reuter, 1905 (Hemiptera: Miridae) / Lívia Aguiar Coelho. – Viçosa, MG, 2012. viii, 132f. : il. ; 29cm. Orientador: Paulo Sérgio Fiuza Ferreira. Tese (doutorado) - Universidade Federal de Viçosa. Inclui bibliografia. 1. Miridae - Classificação. 2. Prepops. 3. Biogeografia. 4. Hemiptera. I. Universidade Federal de Viçosa. II. Título. CDD 22. ed. 595.754 LÍVIA AGUIAR COELHO CONTRIBUIÇÃO À TAXONOMIA E BIOGEOGRAFIA DO GÊNERO Prepops REUTER, 1905 (HEMIPTERA: MIRIDAE) Tese apresentada à Universidade Federal de Viçosa, como parte das exigências do Programa de Pós-Graduação em Entomologia, para obtenção do título de Doctor Scientiae. APROVADA: 25 de fevereiro de 2012. ______________________________ ______________________________ Carlos Molineri David dos Santos Martins ______________________________ ______________________________ Lúcio A. de Oliveira Campos José Eduardo Serrão (Coorientador) ______________________________ Paulo Sérgio Fiuza Ferreira (Orientador) Dedico Ao querido vovô Gil, que infelizmente não me espera mais chegar de viagem com os olhos cheios de lágrimas e de quem não recebo mais os saquinhos com balas e bombons para dividir com meus colegas... E que mesmo sendo um homem muito simples, apoiou meu trabalho e me fez sentir o orgulho que tinha de mim... ii AGRADECIMENTOS Agradeço ao meu orientador, prof. Paulo Sérgio Fiuza Ferreira, pelo entusiasmo em dividir comigo seu enorme conhecimento em taxonomia de Miridae, grupo pelo qual me encantei. -

Invasive Alien Species in Brazilian Ecoregions and Protected Areas Michele De Sá Dechoum1,2, Rafael Barbizan Sühs2, Silvia De Melo Futada3, and Sílvia Renate Ziller4
24 2 Distribution of Invasive Alien Species in Brazilian Ecoregions and Protected Areas Michele de Sá Dechoum1,2, Rafael Barbizan Sühs2, Silvia de Melo Futada3, and Sílvia Renate Ziller4 1 Departamento de Ecologia e Zoologia, Universidade Federal de Santa Catarina, Florianópolis, Santa Catarina, Brazil 2 Programa de pós‐graduação em Ecologia, Universidade Federal de Santa Catarina, Florianópolis, Santa Catarina, Brazil 3 Instituto Socioambiental, São Paulo, São Paulo, Brazil 4 The Horus Institute for Environmental Conservation and Development, Florianopolis, Santa Catarina, Brazil 2.1 Introduction The transposition of biogeographical barriers associated with human activities leads to the intentional and accidental introduction of species, with no sign of saturation in the accu- mulation of species introductions at the global scale (Seebens et al. 2017). Some introduced species overcome environmental barriers and establish populations beyond the point of introduction, being therefore designated as invasive (Richardson et al. 2000). Biological invasions are one of the major threats to biological diversity and to human well‐being (Vilà and Hulme 2017) and considered one of the main drivers of global environmental change in the Anthropocene (Steffen et al. 2011; Capinha et al. 2015). Invasive alien species cause negative environmental impacts in natural ecosystems, with consequences to the conservation of biodiversity and human livelihoods in Brazil (Leão et al. 2011; Souza et al. 2018). Brazil is the fifth largest country and one of the five megabio- diverse countries in the world, housing the highest richness of freshwater, plant, and amphibian species (Mittermeier et al. 1997, 2004) as well as two of the world’s biodiversity hotspots for conservation (Atlantic Forest and Cerrado) (Myers et al. -

1 Atlantic-Primates: a Dataset of Communities And
1 ATLANTIC-PRIMATES: A DATASET OF COMMUNITIES AND 2 OCCURRENCES OF PRIMATES IN THE ATLANTIC FORESTS OF SOUTH 3 AMERICA 4 Laurence Culot1, Lucas Augusto Pereira1, Ilaria Agostini3,4, Marco Antônio Barreto de 5 Almeida5,6, Rafael Souza Cruz Alves2, Izar Aximoff7, Alex Bager8, María Celia 6 Baldovino3,4, Thiago Ribas Bella9, Júlio César Bicca-Marques6, Caryne Braga10, Carlos 7 Rodrigo Brocardo2, Ana Kellen Nogueira Campelo11, Gustavo R. Canale12, Jader da Cruz 8 Cardoso5, Eduardo Carrano13, Diogo Cavenague Casanova14, Camila Righetto Cassano15, 9 Erika Castro8, Jorge José Cherem16, Adriano Garcia Chiarello17, Braz Antonio Pereira 10 Cosenza18, Rodrigo Costa-Araújo19,20, Nilmara Cristina da Silva21, Mario S. Di 11 Bitetti3,4,22, Aluane Silva Ferreira15, Priscila Coutinho Ribas Ferreira23, Marcos de S. 12 Fialho24, Lisieux Franco Fuzessy1, Guilherme Siniciato Terra Garbino25, Francini de 13 Oliveira Garcia27, Cassiano A. F. R. Gatto19, Carla Cristina Gestich27, Pablo Rodrigues 14 Gonçalves10, Nila Rássia Costa Gontijo28, Maurício Eduardo Graipel16,29, Carlos Eduardo 15 Guidorizzi30, Robson Odeli Espíndola Hack31, Gabriela Pacheco Hass6, Renato Richard 16 Hilário32, André Hirsch33, Ingrid Holzmann34, Daniel Henrique Homem14, Hilton 17 Entringer Júnior35, Gilberto Sabino-Santos Júnior36, Maria Cecília Martins Kierulff 37, 18 Christoph Knogge38, Fernando Lima2,39, Elson Fernandes de Lima9,14, Cristiana Saddy 19 Martins39, Adriana Almeida de Lima40, Alexandre Martins41, Waldney Pereira Martins42, 20 Fabiano R. de Melo43,44, Ricardo Melzew45, João Marcelo Deliberador Miranda46,64, 21 Flávia Miranda41, Andréia Magro Moraes2, Tainah Cruz Moreira23, Maria Santina de 22 Castro Morini11, Mariana B. Nagy-Reis47, Luciana Oklander3,48, Leonardo de Carvalho 23 Oliveira15,49,50, Adriano Pereira Paglia51, Anderson Pagoto11, Marcelo Passamani21, 24 Fernando de Camargo Passos46, Carlos A. -

Dinâmica Da Vegetação Do Ultimo Máximo Glacial (21 Ka) E Holoceno Médio (6 Ka): Um Modelo Ecológico De Nicho De Biomas Baseado Em Clima E Solo
DANIEL MEIRA ARRUDA DINÂMICA DA VEGETAÇÃO DO ULTIMO MÁXIMO GLACIAL (21 KA) E HOLOCENO MÉDIO (6 KA): UM MODELO ECOLÓGICO DE NICHO DE BIOMAS BASEADO EM CLIMA E SOLO Tese apresentada à Universidade Federal de Viçosa, como parte das exigências do Programa de Pós-Graduação em Botânica, para obtenção do título de Doctor Scientiae. VIÇOSA MINAS GERAIS – BRASIL 2016 FichaCatalografica :: Fichacatalografica https://www3.dti.ufv.br/bbt/ficha/cadastrarficha/vis... Ficha catalográfica preparada pela Biblioteca Central da Universidade Federal de Viçosa - Câmpus Viçosa T Arruda, Daniel Meira, 1988- A779d Dinâmica da vegetação do Último Máximo Glacial (21 2016 ka) e Holoceno médio (6 ka) : um modelo ecológico de nicho de biomas baseado em clima e solo / Daniel Meira Arruda. - Viçosa, MG, 2016. v, 62f. : il. (algumas color.) ; 29 cm. Inclui anexos. Orientador : Carlos Ernesto Gonçalves R. Schaefer. Tese (doutorado) - Universidade Federal de Viçosa. Inclui bibliografia. 1. Ecologia vegetal. 2. Nicho (Ecologia). 3. Plantas e solos. 4. Solos e clima. 5. Mudanças Climáticas. I. Universidade Federal de Viçosa. Departamento de Biologia Vegetal. Programa de Pós-graduação em Botânica. II. Título. CDD 22. ed. 581.7 2 de 4 09-06-2017 08:18 DANIEL MEIRA ARRUDA DINÂMICA DA VEGETAÇÃO DO ULTIMO MÁXIMO GLACIAL (21 KA) E HOLOCENO MÉDIO (6 KA): UM MODELO ECOLÓGICO DE NICHO DE BIOMAS BASEADO EM CLIMA E SOLO Tese apresentada à Universidade Federal de Viçosa, como parte das exigências do Programa de Pós-Graduação em Botânica, para obtenção do título de Doctor Scientiae. APROVADA: 08 de março de 2016. _____________________________ _____________________________ Ricardo Ribeiro de Castro Solar Markus Gastauer _____________________________ _____________________________ Elpídio Fernandes Filho Marcelo Leandro Bueno _________________________________ Carlos Ernesto Gonçalves R. -

Distribution Extension and Sympatric Occurrence of Gracilinanus Agilis and G
Biota Neotrop., vol. 9, no. 4 Distribution extension and sympatric occurrence of Gracilinanus agilis and G. microtarsus (Didelphimorphia, Didelphidae), with cytogenetic notes Lena Geise1,3 & Diego Astúa2 1Laboratório de Mastozoologia, Departamento de Zoologia, Instituto de Biologia, Universidade do Estado do Rio de Janeiro – UERJ, Rua São Francisco Xavier, 524, Maracanã, CEP 20550-900, Rio de Janeiro, RJ, Brazil 2Laboratório de Mastozoologia, Departamento de Zoologia, Universidade Federal de Pernambuco – UFPE, Av. Prof. Moraes Rego, s/n, Cidade Universitária, CEP 50670-420, Recife, PE, Brazil e-mail: [email protected] 3Corresponding Author: Lena Geise, e-mail: [email protected] GEISE, L. & ASTÚA, D. Distribution extension and sympatric occurrence of Gracilinanus agilis and G. microtarsus (Didelphimorphia, Didelphidae), with cytogenetic notes. Biota Neotrop., 9(4): http://www. biotaneotropica.org.br/v9n4/en/abstract?short-communication+bn01909042009. Abstract: Gracilinanus microtarsus, from the Atlantic Forest and G. agilis, widespread in central Brazil in the Cerrado and in the northeastern Caatinga are two small Neotropical arboreal opossum species not frequently recorded in simpatry. Here we report eight G. agilis specimens from three localities and 17 G. microtarsus, from 10 localities, all in Minas Gerais, Rio de Janeiro and Bahia states. Species proper identification followed diagnostic characters as appearance of dorsum pelage, ocular-mark, ears and tail lengths and size proportion of the posteromedial vacuities in cranium. Chromosomes in metaphases of five specimens were obtained for both species. Our records extend the previous known geographical distribution of G. microtarsus to Chapada Diamantina, in Bahia State and report the occurrence of both species in simpatry. G. microtarsus, in coastal area, was captured in dense ombrophilous and in semideciduous forests, in deciduous seasonal forest and Cerradão in Chapada Diamantina. -

Identification of Critical Areas for Mammal Conservation in the Brazilian Atlantic Forest Biosphere Reserve
Research Letters Natureza & Conservação 9(1):73-78, July 2011 Copyright© 2011 ABECO Handling Editor: Rafael D. Loyola Brazilian Journal of Nature Conservation doi: 10.4322/natcon.2011.009 Identification of Critical Areas for Mammal Conservation in the Brazilian Atlantic Forest Biosphere Reserve Fábio Suzart de Albuquerque1,*, Maria José Teixeira Assunção-Albuquerque2, Lucía Gálvez-Bravo3, Luis Cayuela4, Marta Rueda2 & José María Rey Benayas2 1 Departamento de Ecología, Centro Andaluz de Medio Ambiente, Universidad de Granada, 18006, Granada, Spain 2 Departamento de Ecología, Universidad de Alcalá, 28871, Alcalá de Henares, Madrid, Spain 3 UNGULATA Research group, Instituto de Investigación en Recursos Cinegéticos (CSIC - UCLM - JCCM) Ronda de Toledo, s/n, 13071, Ciudad Real, Spain 4 Área de Biodiversidad y Conservación, Departamento de Biología y Geología, Universidad Rey Juan Carlos, c/ Tulipán, s/n, E-28933, Móstoles, Madrid, Spain Abstract Herein we identified the geographic location of protected areas (PAs) critical for strengthening mammalian conservation in the Brazilian Atlantic Forest Biosphere Reserve (RMBA) by assessing sites of particular importance for mammal diversity using different biodiversity criteria (richness, rarity, vulnerability) and a connectivity index. Although 95% of mammal species were represented by PAs, most of them had less than 10% of their distribution range protected by these areas. A total of 94 critical areas for mammal conservation–representing 49.60% of the total PAs were identified. Most of these areas were located at endangered ecoregions. We recommend that conservationists and policy makers should identify critical areas in order to guarantee biodiversity fluxes among landscapes, and enhance the connectivity between PAs to increase biodiversity protection and conservation. -

In Bahia, Brazil and Diagnosis of Trypanosoma Cruzi Infection
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2009 Ecological risk models for visceral leishmaniais [sic] in Bahia, Brazil and diagnosis of Trypanosoma cruzi infection in dogs in south central Louisiana Prixia Nieto Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Veterinary Pathology and Pathobiology Commons Recommended Citation Nieto, Prixia, "Ecological risk models for visceral leishmaniais [sic] in Bahia, Brazil and diagnosis of Trypanosoma cruzi infection in dogs in south central Louisiana" (2009). LSU Doctoral Dissertations. 859. https://digitalcommons.lsu.edu/gradschool_dissertations/859 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. ECOLOGICAL RISK MODELS FOR VISCERAL LEISHMANIAIS IN BAHIA, BRAZIL AND DIAGNOSIS OF TRYPANOSOMA CRUZI INFECTION IN DOGS IN SOUTH CENTRAL LOUISIANA A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College In partial fulfillment of the Requirements for the degree of Doctor of Philosophy In The Interdepartmental Program in Veterinary Medical Sciences though the Department of Pathobiological Sciences By Prixia Nieto DVM., La Salle University, Colombia, 2001 May 2009 ACKNOWLEDGEMENTS I would deeply like to thank Dr. John B. Malone for his enduring enthusiasm and for believing in me. It has been a pleasure to work with Dr. Maria Emilia Bavia from the Universidade Federal da Bahia, who was very generous for providing me all the information assistance I needed. -
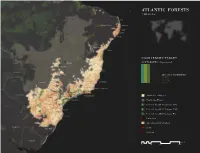
ATLANTIC FORESTS 1,440,959 Km2
ATLANTIC FORESTS 1,440,959 km2 Natal Juazeiro Do Norte João Pessoa Recife Brazil Maceió Aracaju Feira de Santana Salvador BIODIVERSITY TARGET Brasilia 2020 TARGET: 17% protected Goiania Bolivia 2015: 8% PROTECTED 2% I-IV Belo Horizonte 3% V-VI Grande Vitória Camop Grande 3% NA Juiz de Fora Campos dos Goytacazes Volta Redonda Piracicaba Campinas São José Paraguay Jundiaídos Campos Londrina Sorocaba Rio de Janeiro Atlantic Forest Hotspot Maringá São Paulo Neighboring Hotspot Curitiba Ciudad del Este Protected Area (IUCN Category I-IV) Joinville Protected Area (IUCN Category V-VI) Blumenau Protected Area (IUCN Category NA) Florianópolis Urban Area Agriculture (0-100% landuse) Argentina Roads Porto Alegre Railroads Uruguay Kilometers 0 250 500 1,000 ATLANTIC FOREST ECOREGIONS Shortfall Assessment to reach Target of 17% protected land in each terrestrial ecoregion 1 8 14 4 3 5 11 10 12 2 7 13 6 9 15 Kilometers 0 100 200 400 600 800 1,000 Argentina, Brazil, Paraguay 4 BIOMES Deserts & Xeric Shrublands Mangroves Tropical & Subtropical Grasslands, Savannas & Shrublands Tropical & Subtropical Moist Broadleaf Forests 15 ECOREGIONS ENDEMIC PLANT SPECIES 8,000 Kilometers ENDEMIC ANIMAL SPECIES 0 250 500 1,000 725 1. Caatinga Enclaves Moist Forests 5. Campos Rupestres Moist Forests 2,723 km2 remnant habitat 6,592 km2 remnant habitat Target reached Target achieved 2. Bahia Mangroves 563 km2 remnant habitat Target reached 3. Pernambuco Coastal Forests 6. Arucaria Moist Forests 2 842 km remnant habitat 128,274 km2 remnant habitat To reach Aichi Target of 17% + 2,782 km2 protected land To reach Aichi Target of 17% + 38,482 km2 protected land 4. -

Distribution and Causes of Global Forest Fragmentation Page 1 of 11
Conservation Ecology: Distribution and causes of global forest fragmentation Page 1 of 11 Copyright © 2003 by the author(s). Published here under licence by The Resilience Alliance. The following is the established format for referencing this article: Wade, T. G., K. H. Riitters, J. D. Wickham, and K. B. Jones. 2003. Distribution and causes of global forest fragmentation. Conservation Ecology 7(2): 7. [online] URL: http://www.consecol.org/vol7/iss2/art7 Report Distribution and Causes of Global Forest Fragmentation Timothy G. Wade1, Kurt H. Riitters2, James D. Wickham1, and K. Bruce Jones1 1U.S. Environmental Protection Agency, National Exposure Research Laboratory; 2U.S. Forest Service l Abstract l Introduction l Methods l Results l Discussion l Acknowledgments l Responses to this Article l Literature Cited ABSTRACT Because human land uses tend to expand over time, forests that share a high proportion of their borders with anthropogenic uses are at higher risk of further degradation than forests that share a high proportion of their borders with non-forest, natural land cover (e.g., wetland). Using 1-km advanced very high resolution radiometer (AVHRR) satellite-based land cover, we present a method to separate forest fragmentation into natural and anthropogenic components, and report results for all inhabited continents summarized by World Wildlife Fund biomes. Globally, over half of the temperate broadleaf and mixed forest biome and nearly one quarter of the tropical rainforest biome have been fragmented or removed by humans, as opposed to only 4% of the boreal forest. Overall, Europe had the most human-caused fragmentation and South America the least. -

How Effective Is the MERCOSUR's Network of Protected Areas In
Oryx Vol 40 No 1 January 2006 How effective is the MERCOSUR’s network of protected areas in representing South America’s ecoregions? Alvaro Soutullo and Eduardo Gudynas Appendix Southern South American ecoregions within the MERCOSUR and proportion of each ecoregion officially protected. Note that when only the areas in IUCN categories I–IV are considered the proportion of ecoregions protected decreases dramatically. % of areas officially % of areas in % of areas in Ecoregion % of MERCOSUR protected categories I–VI categories I–IV Alta Parana Atlantic forests 3.5 3.6 3.2 1.7 Amapa mangroves 0.0 0.0 0.0 0.0 Araucaria moist forests 1.6 2.3 2.3 0.8 Argentine Espinal 0.8 0.1 0.1 0.0 Argentine Monte 3.0 4.4 4.4 1.0 Arid Chaco 0.7 1.8 1.8 1.6 Atacama desert 0.8 1.0 1.0 1.0 Atlantic Coast restingas 0.1 2.2 2.2 2.1 Atlantic dry forests 0.8 4.6 4.6 4.6 Bahia coastal forests 0.8 1.8 1.3 1.2 Bahia interior forests 1.7 1.6 1.6 1.1 Bahia mangroves 0.0 10.9 0.0 0.0 Beni savannah 0.9 0.0 0.0 0.0 Bolivian montane dry forests 0.6 1.1 1.1 0.2 Bolivian Yungas 0.6 50.6 50.6 27.4 Cordoba montane savannah 0.4 1.2 1.2 0.8 Caatinga 5.4 2.1 2.1 0.8 Caatinga Enclaves moist forests 0.0 7.0 7.0 0.2 Campos Rupestres montane savannah 0.2 19.5 19.5 2.5 Caqueta moist forests 0.1 91.3 1.4 0.0 Central Andean dry puna 2.2 13.6 13.6 6.0 Central Andean puna 0.7 22.8 22.8 6.3 Central Andean wet puna 0.1 3.7 3.7 3.7 Cerrado 14.0 6.6 2.8 2.3 Chaco 4.5 10.6 10.5 5.7 Chilean matorral 1.1 0.9 0.9 0.9 Chiquitano dry forests 1.7 16.2 14.7 0.6 Guayanan Highlands moist forests 0.8 82.8