Expression of Xenobiotic-Metabolizing Enzymes in Human Pulmonary Tissue: Possible Role in Susceptibility for ILD
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Identification and Developmental Expression of the Full Complement Of
Goldstone et al. BMC Genomics 2010, 11:643 http://www.biomedcentral.com/1471-2164/11/643 RESEARCH ARTICLE Open Access Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish Jared V Goldstone1, Andrew G McArthur2, Akira Kubota1, Juliano Zanette1,3, Thiago Parente1,4, Maria E Jönsson1,5, David R Nelson6, John J Stegeman1* Abstract Background: Increasing use of zebrafish in drug discovery and mechanistic toxicology demands knowledge of cytochrome P450 (CYP) gene regulation and function. CYP enzymes catalyze oxidative transformation leading to activation or inactivation of many endogenous and exogenous chemicals, with consequences for normal physiology and disease processes. Many CYPs potentially have roles in developmental specification, and many chemicals that cause developmental abnormalities are substrates for CYPs. Here we identify and annotate the full suite of CYP genes in zebrafish, compare these to the human CYP gene complement, and determine the expression of CYP genes during normal development. Results: Zebrafish have a total of 94 CYP genes, distributed among 18 gene families found also in mammals. There are 32 genes in CYP families 5 to 51, most of which are direct orthologs of human CYPs that are involved in endogenous functions including synthesis or inactivation of regulatory molecules. The high degree of sequence similarity suggests conservation of enzyme activities for these CYPs, confirmed in reports for some steroidogenic enzymes (e.g. CYP19, aromatase; CYP11A, P450scc; CYP17, steroid 17a-hydroxylase), and the CYP26 retinoic acid hydroxylases. Complexity is much greater in gene families 1, 2, and 3, which include CYPs prominent in metabolism of drugs and pollutants, as well as of endogenous substrates. -

Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid
Comprehensive Summaries of Uppsala Dissertations from the Faculty of Pharmacy 231 _____________________________ _____________________________ Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid Cloning, Expression and Catalytic Properties of CYP4F8 and CYP4F21 BY JOHAN BYLUND ACTA UNIVERSITATIS UPSALIENSIS UPPSALA 2000 Dissertation for the Degree of Doctor of Philosophy (Faculty of Pharmacy) in Pharmaceutical Pharmacology presented at Uppsala University in 2000 ABSTRACT Bylund, J. 2000. Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid: Cloning, Expression and Catalytic Properties of CYP4F8 and CYP4F21. Acta Universitatis Upsaliensis. Comprehensive Summaries of Uppsala Dissertations from Faculty of Pharmacy 231 50 pp. Uppsala. ISBN 91-554-4784-8. Cytochrome P450 (P450 or CYP) is an enzyme system involved in the oxygenation of a wide range of endogenous compounds as well as foreign chemicals and drugs. This thesis describes investigations of P450-catalyzed oxygenation of prostaglandins, linoleic and arachidonic acids. The formation of bisallylic hydroxy metabolites of linoleic and arachidonic acids was studied with human recombinant P450s and with human liver microsomes. Several P450 enzymes catalyzed the formation of bisallylic hydroxy metabolites. Inhibition studies and stereochemical analysis of metabolites suggest that the enzyme CYP1A2 may contribute to the biosynthesis of bisallylic hydroxy fatty acid metabolites in adult human liver microsomes. 19R-Hydroxy-PGE and 20-hydroxy-PGE are major components of human and ovine semen, respectively. They are formed in the seminal vesicles, but the mechanism of their biosynthesis is unknown. Reverse transcription-polymerase chain reaction using degenerate primers for mammalian CYP4 family genes, revealed expression of two novel P450 genes in human and ovine seminal vesicles. -

Synonymous Single Nucleotide Polymorphisms in Human Cytochrome
DMD Fast Forward. Published on February 9, 2009 as doi:10.1124/dmd.108.026047 DMD #26047 TITLE PAGE: A BIOINFORMATICS APPROACH FOR THE PHENOTYPE PREDICTION OF NON- SYNONYMOUS SINGLE NUCLEOTIDE POLYMORPHISMS IN HUMAN CYTOCHROME P450S LIN-LIN WANG, YONG LI, SHU-FENG ZHOU Department of Nutrition and Food Hygiene, School of Public Health, Peking University, Beijing 100191, P. R. China (LL Wang & Y Li) Discipline of Chinese Medicine, School of Health Sciences, RMIT University, Bundoora, Victoria 3083, Australia (LL Wang & SF Zhou). 1 Copyright 2009 by the American Society for Pharmacology and Experimental Therapeutics. DMD #26047 RUNNING TITLE PAGE: a) Running title: Prediction of phenotype of human CYPs. b) Author for correspondence: A/Prof. Shu-Feng Zhou, MD, PhD Discipline of Chinese Medicine, School of Health Sciences, RMIT University, WHO Collaborating Center for Traditional Medicine, Bundoora, Victoria 3083, Australia. Tel: + 61 3 9925 7794; fax: +61 3 9925 7178. Email: [email protected] c) Number of text pages: 21 Number of tables: 10 Number of figures: 2 Number of references: 40 Number of words in Abstract: 249 Number of words in Introduction: 749 Number of words in Discussion: 1459 d) Non-standard abbreviations: CYP, cytochrome P450; nsSNP, non-synonymous single nucleotide polymorphism. 2 DMD #26047 ABSTRACT Non-synonymous single nucleotide polymorphisms (nsSNPs) in coding regions that can lead to amino acid changes may cause alteration of protein function and account for susceptivity to disease. Identification of deleterious nsSNPs from tolerant nsSNPs is important for characterizing the genetic basis of human disease, assessing individual susceptibility to disease, understanding the pathogenesis of disease, identifying molecular targets for drug treatment and conducting individualized pharmacotherapy. -

CYP2F1 (NM 000774) Human Tagged ORF Clone Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for RC223097 CYP2F1 (NM_000774) Human Tagged ORF Clone Product data: Product Type: Expression Plasmids Product Name: CYP2F1 (NM_000774) Human Tagged ORF Clone Tag: Myc-DDK Symbol: CYP2F1 Synonyms: C2F1; CYP2F; CYPIIF1 Vector: pCMV6-Entry (PS100001) E. coli Selection: Kanamycin (25 ug/mL) Cell Selection: Neomycin This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 4 CYP2F1 (NM_000774) Human Tagged ORF Clone – RC223097 ORF Nucleotide >RC223097 representing NM_000774 Sequence: Red=Cloning site Blue=ORF Green=Tags(s) TTTTGTAATACGACTCACTATAGGGCGGCCGGGAATTCGTCGACTGGATCCGGTACCGAGGAGATCTGCC GCCGCGATCGCC ATGGACAGCATAAGCACAGCCATCTTACTCCTGCTCCTGGCTCTCGTCTGTCTGCTCCTGACCCTAAGCT CAAGAGATAAGGGAAAGCTGCCTCCGGGACCCAGACCCCTCTCAATCCTGGGAAACCTGCTGCTGCTTTG CTCCCAAGACATGCTGACTTCTCTCACTAAGCTGAGCAAGGAGTATGGCTCCATGTACACAGTGCACCTG GGACCCAGGCGGGTGGTGGTCCTCAGCGGGTACCAAGCTGTGAAGGAGGCCCTGGTGGACCAGGGAGAGG AGTTTAGTGGCCGCGGTGACTACCCTGCCTTTTTCAACTTTACCAAGGGCAATGGCATCGCCTTCTCCAG TGGGGATCGATGGAAGGTCCTGAGACAGTTCTCTATCCAGATTCTACGGAATTTCGGGATGGGGAAGAGA AGCATTGAGGAGCGAATCCTAGAGGAGGGCAGCTTCCTGCTGGCGGAGCTGCGGAAAACTGAAGGCGAGC CCTTTGACCCCACGTTTGTGCTGAGTCGCTCAGTGTCCAACATTATCTGTTCCGTGCTCTTCGGCAGCCG CTTCGACTATGATGATGAGCGTCTGCTCACCATTATCCGCCTTATCAATGACAACTTCCAAATCATGAGC -

Robert Foti to Cite This Version
Characterization of xenobiotic substrates and inhibitors of CYP26A1, CYP26B1 and CYP26C1 using computational modeling and in vitro analyses Robert Foti To cite this version: Robert Foti. Characterization of xenobiotic substrates and inhibitors of CYP26A1, CYP26B1 and CYP26C1 using computational modeling and in vitro analyses. Agricultural sciences. Université Nice Sophia Antipolis, 2016. English. NNT : 2016NICE4033. tel-01376678 HAL Id: tel-01376678 https://tel.archives-ouvertes.fr/tel-01376678 Submitted on 5 Oct 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Université de Nice-Sophia Antipolis Thèse pour obtenir le grade de DOCTEUR DE L’UNIVERSITE NICE SOPHIA ANTIPOLIS Spécialité : Interactions Moléculaires et Cellulaires Ecole Doctorale : Sciences de la Vie et de la Santé (SVS) Caractérisation des substrats xénobiotiques et des inhibiteurs des cytochromes CYP26A1, CYP26B1 et CYP26C1 par modélisation moléculaire et études in vitro présentée et soutenue publiquement par Robert S. Foti Le 4 Juillet 2016 Membres du jury Dr. Danièle Werck-Reichhart Rapporteur Dr. Philippe Roche Rapporteur Pr. Serge Antonczak Examinateur Dr. Philippe Breton Examinateur Pr. Philippe Diaz Examinateur Dr. Dominique Douguet Directrice de thèse 1 1. Table of Contents 1. Table of Contents .............................................................................................................................. -

Metabolic Activation and Toxicological Evaluation of Polychlorinated Biphenyls in Drosophila Melanogaster T
www.nature.com/scientificreports OPEN Metabolic activation and toxicological evaluation of polychlorinated biphenyls in Drosophila melanogaster T. Idda1,7, C. Bonas1,7, J. Hofmann1, J. Bertram1, N. Quinete1,2, T. Schettgen1, K. Fietkau3, A. Esser1, M. B. Stope4, M. M. Leijs3, J. M. Baron3, T. Kraus1, A. Voigt5,6 & P. Ziegler1* Degradation of polychlorinated biphenyls (PCBs) is initiated by cytochrome P450 (CYP) enzymes and includes PCB oxidation to OH-metabolites, which often display a higher toxicity than their parental compounds. In search of an animal model refecting PCB metabolism and toxicity, we tested Drosophila melanogaster, a well-known model system for genetics and human disease. Feeding Drosophila with lower chlorinated (LC) PCB congeners 28, 52 or 101 resulted in the detection of a human-like pattern of respective OH-metabolites in fy lysates. Feeding fies high PCB 28 concentrations caused lethality. Thus we silenced selected CYPs via RNA interference and analyzed the efect on PCB 28-derived metabolite formation by assaying 3-OH-2′,4,4′-trichlorobiphenyl (3-OHCB 28) and 3′-OH-4′,4,6′-trichlorobiphenyl (3′-OHCB 28) in fy lysates. We identifed several drosophila CYPs (dCYPs) whose knockdown reduced PCB 28-derived OH-metabolites and suppressed PCB 28 induced lethality including dCYP1A2. Following in vitro analysis using a liver-like CYP-cocktail, containing human orthologues of dCYP1A2, we confrm human CYP1A2 as a PCB 28 metabolizing enzyme. PCB 28-induced mortality in fies was accompanied by locomotor impairment, a common phenotype of neurodegenerative disorders. Along this line, we show PCB 28-initiated caspase activation in diferentiated fy neurons. -

Strong Correlation Between Air-Liquid Interface Cultures and in Vivo Transcriptomics of Nasal Brush Biopsy
Am J Physiol Lung Cell Mol Physiol 318: L1056–L1062, 2020. First published April 1, 2020; doi:10.1152/ajplung.00050.2020. RAPID REPORT Translational Physiology Strong correlation between air-liquid interface cultures and in vivo transcriptomics of nasal brush biopsy X Baishakhi Ghosh,1 Bongsoo Park,1 Debarshi Bhowmik,2 Kristine Nishida,3 Molly Lauver,3 Nirupama Putcha,3 Peisong Gao,4 Murugappan Ramanathan, Jr.,5 Nadia Hansel,3 Shyam Biswal,1 and Venkataramana K. Sidhaye1,3 1Department of Environmental Health and Engineering, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland; 2Department of Biology, Johns Hopkins University, Baltimore, Maryland; 3Department of Pulmonary and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland; 4Division of Allergy and Clinical Immunology, Johns Hopkins School of Medicine, Baltimore, Maryland; and 5Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins School of Medicine, Baltimore, Maryland Submitted 18 February 2020; accepted in final form 24 March 2020 Ghosh B, Park B, Bhowmik D, Nishida K, Lauver M, Putcha N, RNA sequencing to determine the correlation between ALI- Gao P, Ramanathan M Jr, Hansel N, Biswal S, Sidhaye VK. cultured epithelial cells and epithelial cells obtained from nasal Strong correlation between air-liquid interface cultures and in vivo brushing and identify differences that may arise as a result of transcriptomics of nasal brush biopsy. Am J Physiol Lung Cell Mol redifferentiation. Physiol 318: L1056–L1062, 2020. First published April 1, 2020; doi:10.1152/ajplung.00050.2020.—Air-liquid interface (ALI) cultures METHODS are ex vivo models that are used extensively to study the epithelium of patients with chronic respiratory diseases. -

Metabolic Activation of 4-Ipomeanol by Complementary DNA-Expressed Human Cytochromes P-450: Evidence for Species-Specific Metabolism Maciej Czerwinski, Theodore L
[CANCER RESEARCH 51. 4636-4638, September I, 1991] Metabolic Activation of 4-Ipomeanol by Complementary DNA-expressed Human Cytochromes P-450: Evidence for Species-specific Metabolism Maciej Czerwinski, Theodore L. McLemore,1 Richard M. Philpot, Patson T. Nhamburo, Kenneth Korzekwa, Harry V. Gelboin, and Frank J. Gonzalez Laboratory of Molecular Carcinogenesis, Division of Cancer Etiology [M. C., P. T. N., K. K., H. V. G., F. J. G.¡,and Developmental Therapeutics Program, Division of Cancer Treatment [T. L. M.], National Cancer Institute, NIH, Bethesda, Maryland 20892, and Laboratory of Pharmacology, National Institutes of Environmental Health Sciences, NIH, Research Triangle Park, North Carolina 27709 ¡R.M. P.] ABSTRACT Specific enzymes present in human lung that are capable of activating 4-ipomeanol have not been established previously. 4-Ipomeanol is a pulmonary toxin in cattle and rodents that is meta- We therefore studied the ability of 14 P-450s to activate 4- bolically activated by cytochromes P-450 (P-450s). P-450-mediated ac ipomeanol to DNA-binding metabolites using vaccinia virus- tivation of 4-ipomeanol to DNA binding metabolites was evaluated using mediated cDNA expression of P-450s in human Hep G2 cells a vaccinia virus complementary DNA expression system and an in situ DNA-binding assay. Twelve human P-450s and two rodent P-450s were and in situ binding to cellular DNA. We report that several P- expressed in human hepatoma Hep G2 cells and examined for their 450s can catalyze some activation of 4-ipomeanol and at least abilities to metabolically activate this toxin. Three forms, designated three distinct human P-450 forms are capable of high rates of CYP1A2, CYP3A3, and CYP3A4, were able to catalyze significant biotransformation of this compound. -

Expression of CYP2S1 in Human Hepatic Stellate Cells
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector FEBS Letters 581 (2007) 781–786 Expression of CYP2S1 in human hepatic stellate cells Carylyn J. Mareka, Steven J. Tuckera, Matthew Korutha, Karen Wallacea,b, Matthew C. Wrighta,b,* a School of Medical Sciences, Institute of Medical Science, University of Aberdeen, Aberdeen, UK b Liver Faculty Research Group, School of Clinical and Laboratory Sciences, University of Newcastle, Newcastle, UK Received 22 November 2006; revised 16 January 2007; accepted 23 January 2007 Available online 2 February 2007 Edited by Laszlo Nagy the expression and accumulation of scarring extracellular Abstract Activated stellate cells are myofibroblast-like cells associated with the generation of fibrotic scaring in chronically fibrotic matrix protein [2]. It is currently thought an inhibition damaged liver. Gene chip analysis was performed on cultured fi- of fibrosis in liver diseases may be an effective approach to brotic stellate cells. Of the 51 human CYP genes known, 13 treating patients for which the cause is refractive to current CYP and 5 CYP reduction-related genes were detected with 4 treatments (e.g. in approx. 30% of hepatitis C infected CYPs (CYP1A1, CYP2E1, CY2S1 and CYP4F3) consistently patients) [2,3]. At present, there is no approved treatment for present in stellate cells isolated from three individuals. Quantita- fibrosis. tive RT-PCR indicated that CYP2S1 was a major expressed Inadvertent toxicity of drugs is often associated with a ‘‘met- CYP mRNA transcript. The presence of a CYP2A-related pro- abolic activation’’ by CYPs [1]. -

Recent Advances in P450 Research
The Pharmacogenomics Journal (2001) 1, 178–186 2001 Nature Publishing Group All rights reserved 1470-269X/01 $15.00 www.nature.com/tpj REVIEW Recent advances in P450 research JL Raucy1,2 ABSTRACT SW Allen1,2 P450 enzymes comprise a superfamily of heme-containing proteins that cata- lyze oxidative metabolism of structurally diverse chemicals. Over the past few 1La Jolla Institute for Molecular Medicine, San years, there has been significant progress in P450 research on many fronts Diego, CA 92121, USA; 2Puracyp Inc, San and the information gained is currently being applied to both drug develop- Diego, CA 92121, USA ment and clinical practice. Recently, a major accomplishment occurred when the structure of a mammalian P450 was determined by crystallography. Correspondence: Results from these studies will have a major impact on understanding struc- JL Raucy,La Jolla Institute for Molecular Medicine,4570 Executive Dr,Suite 208, ture-activity relationships of P450 enzymes and promote prediction of drug San Diego,CA 92121,USA interactions. In addition, new technologies have facilitated the identification Tel: +1 858 587 8788 ext 116 of several new P450 alleles. This information will profoundly affect our under- Fax: +1 858 587 6742 E-mail: jraucyȰljimm.org standing of the causes attributed to interindividual variations in drug responses and link these differences to efficacy or toxicity of many thera- peutic agents. Finally, the recent accomplishments towards constructing P450 null animals have afforded determination of the role of these enzymes in toxicity. Moreover, advances have been made towards the construction of humanized transgenic animals and plants. Overall, the outcome of recent developments in the P450 arena will be safer and more efficient drug ther- apies. -

PDF (Supplementary Figures)
Supplementary Information Insights into an Efficient Light-driven Hybrid P450 BM3 Enzyme from Crystallographic, Spectroscopic and Biochemical Studies Jessica Spradlin,1 Diana Lee,1 Sruthi Mahadevan,1 Mavish Mahomed,2 Lawrence Tang,1 Quan Lam,1 Alexander Colbert,1 Oliver S. Shafaat,3 David Goodin,2 Marco Kloos,4 Mallory Kato,1 Lionel E. Cheruzel1* 1 San José State University, Department of Chemistry, One Washington Square, San José, CA 2 Department of Chemistry, One Shields Ave., University of California Davis, Davis, CA. 3 Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA. 4 Department of Biomolecular Mechanisms, Max Planck Institute for Medical Research, Heidelberg, Germany. Table of Contents: Figure S1. Graphical representations of C-H---pi interactions with P382..……………………………p.S-2 Figure S2. Transient absorption traces for the various hybrid enzymes …………….………………...p.S-3 Figure S3. UV vis spectra for the substrate-free, NPG bound and reduced hybrid enzymes..….……..p.S-4 Figure S4. Michaelis-Menten saturation curves with 16-pNCA substrate………….…………...……..p.S-4 Figure S5. Partial sequence alignment of 40 human cytochrome P450 heme domains….……...……..p.S-5 S-1 Fig. S1. Graphical representations of C-H---π interactions between the photosensitizer and the P382 residue preserved during the motion of the 310 helix by 2.1 Å in the two molecules present in the asymmetric unit of the DMSO bound structure. S-2 Fig. S2. Transient absorption traces for the dF393A-1, dF393W-1 and sL407C-1 hybrid enzymes and the corresponding difference spectra for the substrate free (SF) and substrate bound (SB) forms (bottom panels). -
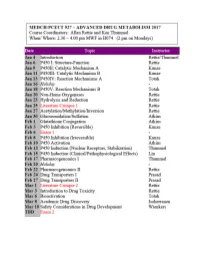
CYTOCHROME P450: Structure-Function
MEDCH 527 AER Jan. 4-6, 2017 CYTOCHROME P450: Structure-Function 1. General P450 Characteristics anD Taxonomy 2. Human P450s – Substrate anD Inhibitor Selectivities 3. Structure-Function Aspects of LiganD BinDing, P450 ReDuction anD Oxygen Activation References P450 Homepage -http:// http://drnelson.uthsc.edu/CytochromeP450.html Nelson DR et al., Pharmacogenetics. 1996 6(1):1-42: Nelson DR et al., Pharmacogenetics. 2004 14(1):1-18. Testa, B. The Biochemistry of Drug Metabolism: A 6 Part Series in Chem. BioDivers. (2006-2008). Sligar, SG. Glimpsing the critical intermediate in cytochrome P450 oxidations. Science. 2010 Nov 12;330(6006):924-5. Johnson EF, et al. Correlating structure and function of drug metabolizing enzymes:Progress and ongoing challenges. Drug Metab. Dispos. 42:9-22 (2014). Zientek MA and Youdim K, Reaction Phenotyping:Advances in experimental strategies used to characterize the contribution of drug metabolizing enzymes. Drug Metab. Dispos. 43:163-181 (2015). Almazroo et al., Drug Metabolism in the Liver. Clin. Liver Dis. 21:1-20 (2017). Absorption, Distribution, Metabolism and Excretion (ADME) of Orally Administered Drugs To reach their sites of action in the body, orally administered drugs must be absorbed from the small intestine, survive first pass metabolism - typically in the liver - before eventually being excreted, usually as drug metabolites in the bile and kidney. Therefore, clinically useful drugs must be able to cross an array of cell membranes, which are composed of a lipid bilayer. Drugs must exhibit an adequate degree of lipophilicity (logP of ~2-4) in order to able to dissolve into this lipoidal environment. Many drug metabolism processes render lipophilic drugs more water-soluble so as to facilitate excretion via the kidneys and bile.