Genitourinary Tract Infections
Total Page:16
File Type:pdf, Size:1020Kb
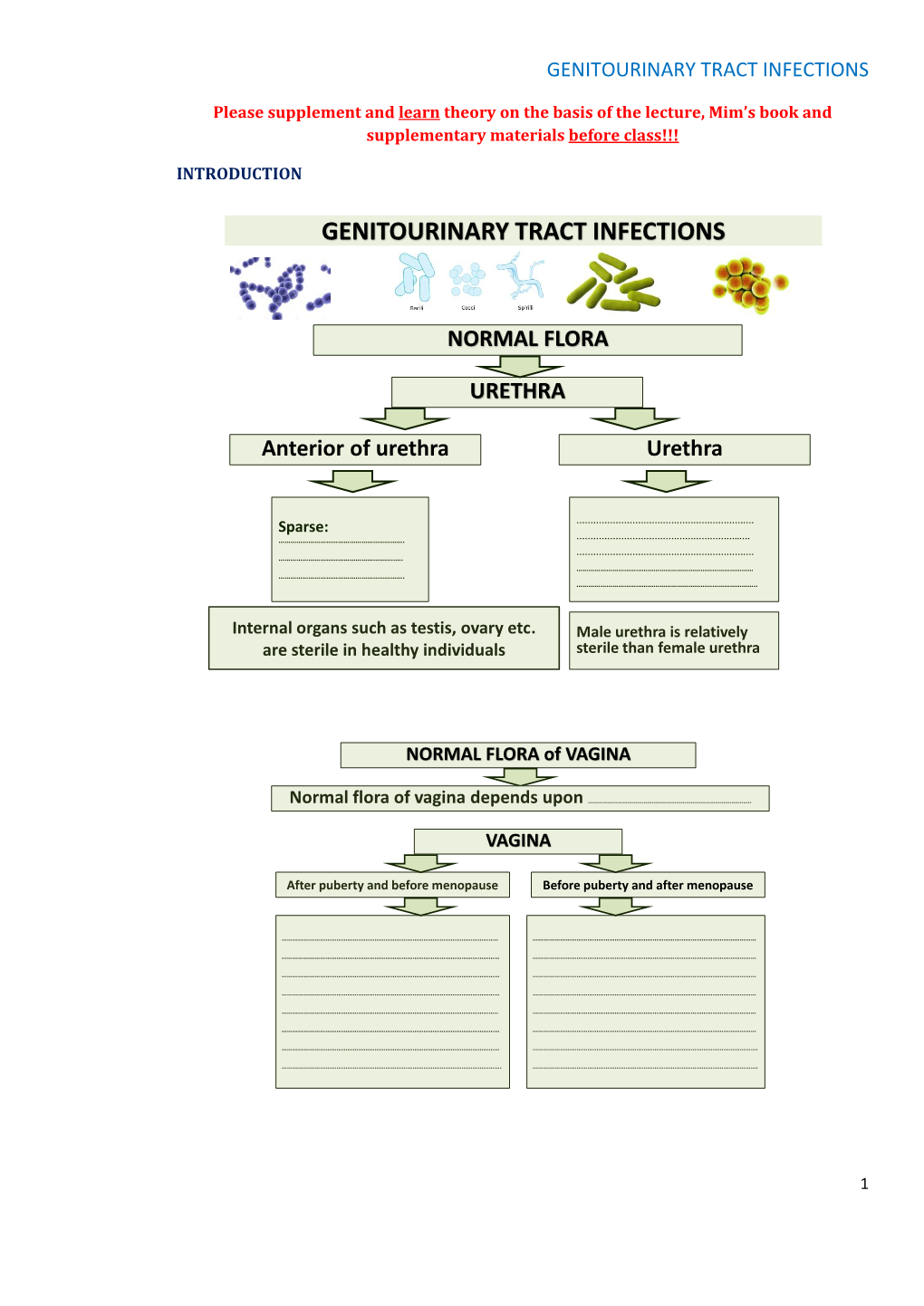
Load more
Recommended publications
-

Cervical Dysplasia, Ploidy, and Human Papillomavirus Status Correlate with Loss of Fhit Expression1
1306 Vol. 7, 1306–1312, May 2001 Clinical Cancer Research Cervical Dysplasia, Ploidy, and Human Papillomavirus Status Correlate with Loss of Fhit Expression1 Andrea Vecchione, Nicola Zanesi, Conclusions: These data clearly suggest that loss of Fhit Giorgio Trombetta, Debora French, Paolo Visca, expression occurs in the early stages of cervical carcinogen- esis. Pap test represents one of the most convenient and Tiziana Pisani, Claudio Botti, Aldo Vecchione, 2 rapid procedures available in identification of cellular Carlo M. Croce, and Rita Mancini changes; hence, Fhit staining might be used as an useful tool Department of Microbiology and Immunology, Kimmel Cancer in larger population screening to detect early alteration in Center, Thomas Jefferson University, Philadelphia, Pennsylvania cellular behaviors. 19107 [A. V., N. Z., C. M. C., R. M.]; Department of Experimental Medicine and Pathology, University “La Sapienza,” Rome, Italy [A. V.]; Centro Ricerche “Ospedale S. Pietro” [D. F., A. V., R. M.] INTRODUCTION and II University “La Sapienza” [T. P., A. V.], Rome, Italy; and Cervical carcinoma is one of the most deadly neoplasms in Regina Elena National Cancer Institute, Rome, Italy 00161 [G. T., P. V., C. B.] women, particularly in developing countries (1). The relation- ship between HPV3 infection and precancerous cervical lesions, such as LGSILs and HGSILs, has been demonstrated clearly ABSTRACT (2–4). Cytomorphological identification of cellular changes is Purpose: The tumor suppressor gene, FHIT, has been currently the most convenient, rapid, economical, and sensitive cloned and mapped at chromosome region 3p14.2, one of the procedure available for detection of HPV infection in the genital regions most frequently deleted in cervical carcinoma. -

Koilocytes, Papanicolaou, Endocervical, Dysplasia, CD4, CD8
American Journal of Medicine and Medical Sciences 2012, 2(4): 75-79 DOI: 10.5923/j.ajmms.20120204.03 Correlation Between Koilocytes, HPV DNA and CD4 Cells Count in Male and Female HIV Cohorts Ajobiewe Olu Joseph1,*, Isu Nnena Rosemary1, Agwale Simon2 1University of Abuja, Department of Biological Sciences, Faculty of Natural Sciences, PMB 117, Gwagwalada, Federal Capital Territory (F.C.T.) Abuja Nigeria 2Innovative biotechnology Inc3516,Langrehr Road, Suite 2A, Windsor M.ill. MD 21244, USA Abstract The study x-rays the relationship between Koilocytes and [Cluster of Differentiation]CD4 Count from HIV positive unisex cohorts aged 15 -40 years and above. The cohorts, were randomly tested for the presence of koilocytes, using their semen and endocervical swab samples respectively at some top Hospitals in Abuja, F.C.T. Nigeria. Their blood samples were tested for CD4 count. Papanicolaou staining technique was adopted-for demonstrating koilocytes. Results showed that, high koilocytes count of 39.0% and low CD4 Count of 350 lymphocyte counts /µl were observed; and low monthly mean koilocytes count of 7.60% and high CD4 Count of 687 lymphocytes counts/ µl were observed in the female and male cohorts respectively. High monthly prevalence count of koilocytes and low CD4 Count in the female cohorts and verse versa in the male cohorts were significantly correlated (P<0.05). Depression of immunity, is worsen in HPV and HIV co- infectivity. Keywords Koilocytes, Papanicolaou, Endocervical, Dysplasia, CD4, CD8 immunocompromised patients had decreased plasma and 1. Study Background Langerhans cells in the cervix, but increased T cells. In ad- dition there was inversion of the CD4: CD8 ratio inde- HPV does not disseminate and thus the local cervical pendent of the systemic CD4 counts. -

Association of Human Papilloma Virus with Oral Squamous Cell Carcinoma Among Bangladeshi Patients
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 18, Issue 11 Ser.12 (November. 2019), PP 41-46 www.iosrjournals.org Association of Human Papilloma Virus with Oral Squamous Cell Carcinoma among Bangladeshi Patients Mir Nowazesh Ali1, RezwanaBinte Anwar2, HumayraBinte Anwar3, Md. Al- Amin Bhuiyan4,Afzalun Nessa5, Md. Abdullah Yusuf6, QuaziBillur Rahman7 1. Mir Nowazesh Ali, Assistant ProfessorOral & Maxillofacial Surgery Department, Bangabandhu Sheikh Mujib Medical University, Shahbagh, Dhaka, Bangladesh; Email: 2. RezwanaBinte Anwar, Medical Officer, Department of Prosthodontics, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh; 3. HumayraBinte Anwar, Research Associate, James P Grant School of Public Health, BRAC University, Dhaka, Bangladesh; 4. Md. Al-Amin Bhuiyan, Project Manager, Centre for Injury Prevention and Research, Bangladesh (CIPRB), Dhaka, Bangladesh; 5. AfzalunNessa, Department of Virology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh; 6. Md. Abdullah Yusuf, Assistant Professor, Department of Microbiology, National Institute of Neurosciences & Hospital, Dhaka, Bangladesh; 7. QuaziBillur Rahman, Chairman & Head of the Department, Oral & Maxillofacial Surgery Department, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh; Correspondence: Dr. Mir Nowazesh Ali, BDS, MS, PhD, Assistant Professor Oral & Maxillofacial Surgery Department, Bangabandhu Sheikh Mujib Medical University, Shahbagh, Dhaka, Bangladesh; Conflict of Interest: There is no financial conflict of interest. Abstract: Background: Cancer is a major challenge for our society today. Oral squamous cell carcinomas might be related to human papilloma virus infection. Objective: The purpose of the present study was to find the association of Human Papilloma virus (HPV) and oral squamous cell carcinomain Bangladesh. Methodology: This analytical cross sectional study was conducted at the Bangabandhu Sheikh Mujib Medical University (BSMMU), Bangladesh and at the University of Dhaka, for one year. -

PAPILLOMAVIRUS-ASSOCIATED FOCAL ORAL HYPERPLASIA in WILD and CAPTIVE ASIAN LIONS Ipanthera LEO PÉRSICA)
Journal of Zoo and Wildlife Medicine 27(1): 61-70, 1996 Copyrighl 1996 by American Association of Zoo Veterinarians PAPILLOMAVIRUS-ASSOCIATED FOCAL ORAL HYPERPLASIA IN WILD AND CAPTIVE ASIAN LIONS iPANTHERA LEO PÉRSICA) John P. Sundberg, D.V.M., Ph.D., Richard J. Montali, D.V.M., Mitchell Bush, D.V.M., Lyndsay G. Phillips, Jr., D.V.M., Stephen J. O'Brien, Ph.D., A. Bennett Jenson, M.D., Robert D. Burk, M.D., and Marc Van Ranst, M.D. Abstract: Four Asian lions (Panthera leo pérsica), two wild and two captive, were diagnosed with focal oral hyperplasia affecting the ventral surface of their tongues. Focal, flat, sessile lesions consisted of hyperplastic, stratified squamous epithelium. Koilocytotic atypia was evident in the upper layers of cells, some of which contained characteristic intranuclear papillomavirus particles visible by electron microscopy. In addition, large amphophilic cytoplasmic inclusions were evident in the koilocytes and were considered to be a product of the viral E4 gene. Papillomavirus group- specific antigens were detected by immunohistochemistry in the atypical cell nuclei. Conserved papillomavirus antigenic epitopes differed from epitopes found in cutaneous papillomavirus-induced lesions from domestic cats. An 8,000-base pair DNA fragment, linearized by Bam HI digestion, was detected by Southern blot hybridization probed with a mixed human papillomavirus genomic probe. Limited restriction endonuclease studies of DNA prepared using an oral hyperplastic lesion from an Asian lion indicate that this is a novel feline papillomavirus different from the domestic cat cutaneous papillomavirus. This new virus has been designated "PIPV." Key words: Papillomavirus, Asian lion, Panthera leo pérsica, felidae, PIPV. -

Stratification of HPV-Induced Cervical Pathology Using the Virally Encoded
Modern Pathology (2015) 28, 977–993 © 2015 USCAP, Inc All rights reserved 0893-3952/15 $32.00 977 Stratification of HPV-induced cervical pathology using the virally encoded molecular marker E4 in combination with p16 or MCM Heather Griffin1,2, Yasmina Soneji2, Romy Van Baars3, Rupali Arora4, David Jenkins3, Miekel van de Sandt3, Zhonglin Wu2, Wim Quint3, Robert Jach5, Krzysztof Okon5, Hubert Huras5, Albert Singer4 and John Doorbar1,2 1Department of Pathology, University of Cambridge, Cambridge, UK; 2National Institute for Medical Research, London, UK; 3DDL Diagnostic Laboratory, Rijswijk, The Netherlands; 4University College Hospital, London, UK and 5Department of Gynecology and Oncology, Jagiellonian University College, Krakow, Poland High-risk human papillomavirus (HPV) types cause cervical lesions of varying severity, ranging from transient productive infections to high-grade neoplasia. Disease stratification requires the examination of lesional pathology, and possibly also the detection of biomarkers. P16INK4a and MCM are established surrogates of high- risk HPV E6/E7 activity, and can be extensively expressed in high-grade lesions. Here we have combined these two cellular biomarkers with detection of the abundant HPV-encoded E4 protein in order to identify both productive and transforming lesions. This approach has allowed us to distinguish true papillomavirus infections from similar pathologies, and has allowed us to divide the heterogeneous CIN2 category into those that are CIN1- like and express E4, and those that more closely resemble nonproductive CIN3. To achieve this, 530 lesional areas were evaluated according to standard pathology criteria and by using a multiple staining approach that allows us to superimpose biomarker patterns either singly or in combination onto an annotated hematoxylin and eosin (H&E) image. -

Essentials of Pap Smear and Breast Cytology
Essentials of Pap Smear and Breast Cytology Brenda Smith Gia-Khanh Nguyen 2012 Essentials of Pap Smear and Breast Cytology Brenda Smith, BSc, RT, CT (ASCP) Clinical Instructor Department of Pathology & Laboratory Medicine University of British Columbia Vancouver, British Columbia, Canada And Gia-Khanh Nguyen, MD, FRCPC Professor Emeritus Department of Laboratory Medicine & Pathology University of Alberta Edmonton, Alberta, Canada All rights reserved. Legally deposited at Library and Archives Canada. ISBN: 978-0- 9780929-7-9. 2 Table of contents Preface 4 Acknowledgements and Related material by the same author 5 Abbreviations and Remarks 6 Chapter 1. Pap smear: An overview 7 Chapter 2. Pap smear: Normal uterus and vagina 18 Chapter 3. Pap smear: Negative for intraepithelial lesion or malignancy: Infections and nonneoplastic findings 28 Chapter 4. Pap smear: Squamous cell abnormalities 51 Chapter 5. Pap smear: Glandular cell abnormalities 69 Chapter 6. Pap smear: Other malignant tumors 90 Chapter 7. Anal Pap smear: Anal-rectal cytology 98 Chapter 8. Breast cytology: An overview 102 Chapter 9. Nonneoplastic breast lesions 106 Chapter10. Breast neoplasms 116 The authors 146 3 Preface This monograph “Essentials of Pap Smear and Breast Cytology” is prepared at the request of a large number of students in cytology who wish to have a small and concise book with numerous illustrations for easy reference during their laboratory training. Most information and illustrations in this book are extracted from the authors’ monograph entitled “Essentials of Gynecologic Cytology”, and they are rearranged according to The Bethesda System-2001. This book should be used in conjunction with the above-mentioned book on gynecologic cytology. -

Human Papillomavirus Infection and P16 Expression in The
Švajdler et al. Diagnostic Pathology (2016) 11:53 DOI 10.1186/s13000-016-0505-3 RESEARCH Open Access Human papillomavirus infection and p16 expression in the immunocompetent patients with extragenital/extraungual Bowen’s disease Marián Švajdler Jr1,2*†, Roman Mezencev3†, Jana Kašpírková2, Denisa Kacerovská1,2, Dmitry V. Kazakov1,2, Ondrej Ondič1,2 and Michal Michal1,2 Abstract Background: The role of human papillomaviruses (HPV) in the development of squamous cell carcinoma (SCC) has been established for anogenital lesions but still remains controversial for carcinomas in other sites. The aim of this study was to determine the α-HPV and β-HPV prevalence and their association with p16 expression, sun exposure, and clinicopathological findings in patients with Bowen’s disease (BD). Methods: One hundred sixty nine skin biopsy specimens from 157 immunocompetent patients with extragenital/extraungual BD were examined for HPV status and p16 expression. The presence of koilocyte-like changes, solar elastosis and papillomatosis was recorded for each specimen. Results: BD was diagnosed more often in potentially sun-exposed sites with prevalence 73.6 % and a remarkable predilection for the head and neck region. High risk α-HPV or β-HPV were detected in 34.7 % of lesions and β-HPV infections dominated over α-HPV. Higher prevalence of koilocyte-like changes and papillomatosis was found in HPV-positive specimens but it was not statistically significant. The expression of p16 was detected in 79.8 % of lesions and displayed no correlation with the HPV status. HPV-positivity tended to be detected more often in sun-protected sites. Dual infections by α-HPV/β-HPV genera and mixed α-HPV infections were not detected, while 37.5 % of β-HPV positive specimens were infected by two or more β-HPV genotypes. -

1 MECHANISMS of HUMAN DISEASE: LABORATORY SESSION CYTOPATHOLOGY Monday, April 26, 2010 GOAL: 1. Understated the Role of Cytopat
MECHANISMS OF HUMAN DISEASE: LABORATORY SESSION CYTOPATHOLOGY Monday, April 26, 2010 FACULTY COPY GOAL: 1. Understated the role of cytopathology in the clinical management of the patient and recognize the utility of various cytology techniques. OBJECTIVES: 1. Describe the Papanicolaou test 2. Describe the cytologic characteristics of HPV infection in a cervical PAP test 3. Describe the role of cytology in diagnosing malignant tumors of the bladder 4. Describe how cytology affects ovarian carcinoma staging 5. Describe the process of a fine needle aspiration biopsy and its advantages 6. Describe the cytologic features of papillary thyroid carcinoma 7. Understand the molecular basis of treatment of GIST What is cytopathology? Cytopathology is a branch of pathology that studies and diagnoses diseases on the cellular level. The most common use of cytopathology is the Pap smear, used to detect cervical cancer at an early treatable stage. Other type of specimens: – exfoliated cells: urines, fluids – washings/brushings: bronchi, renal pelvis, esophagus – fine needle aspirations 1 CASE 1 CHIEF COMPLAINT: Annual routine physical examination. HISTORY: The patient is a 24 year-old sexually active female, with a history of several partners. She feels well. PHYSICAL EXAMINATION: Heart, lung, abdominal exams are normal. Breast exam is normal and without masses. Pelvic exam is unremarkable. A Pap test is obtained. 1. What are the possible Pap test interpretations in this patient? a. Evidence of inflammation b. Sexually transmitted diseases – Herpes, Trichomonas c. Squamous intraepithelial lesion d. Carcinoma e. Normal findings 2. Identify and describe characteristic pathologic findings seen on the Pap test. Koilocyte: Pathognomonic for HPV (human papillomavirus) infection; causes a cytopathic effect in the squamous cells and results in the formation of a characteristic large halo surrounding the nucleus. -

Koilocytes in Oral Pathologies REVIEW ARTICLE
WJD 10.5005/jp-journals-10015-1525Koilocytes in Oral Pathologies REVIEW ARTICLE Koilocytes in Oral Pathologies 1Preeti Singh, 2Samudrala V Sowmya, 3Roopa S Rao, 4Dominic Augustine, 5Vanishree C Haragannavar, 6Shwetha Nambiar ABSTRACT How to cite this article: Singh P, Sowmya SV, Rao RS, Augustine D, Haragannavar VC, Nambiar S. Koilocytes in Oral Introduction: Oral HPV infections affect 1 to 50% of the Pathologies. World J Dent 2018;9(2):149-153. general population. Naturally, about 90% of HPV is eliminated by the immune system, but the ones that persist may result in Source of support: Nil serious diseases. Human papillomavirus is the main cause of Conflict of interest: None cancer at various body sites, such as cervix, uterus, orophar- ynx, head and neck. The prevalence of oral HPV infections in India ranges from 15 to 16%. About 80% of HPV infections are INTRODUCTION present with koilocytosis as an important morphological marker. Human papillomavirus belongs to the family of viruses, Aim: This review focuses on the importance of koilocytes and the Papovaviridae.1 These are small, epitheliotropic its early detection to alert malignant risk for facilitating human double-stranded DNA viruses with more than 120 papillomavirus (HPV)-targeted therapeutic strategies. identified genotypes in human.1,2 There are specific Results: Research in the past has primarily focused on HPV types responsible for the causation of cancerous cervical cancer, as >99% of them harbor HPV. It has been and non-cancerous tumors.3,4 Human papillomavirus observed that the incidence of HPV-associated cancers may be minimized by effective preventive and targeted therapeutic prevalence ranges from 22 to 60% in the normal mucosa modalities. -

Low-Grade Squamous Intraepithelial Lesion and Mimics
Low-Grade Squamous Intraepithelial Lesion and Mimics KEY FACTS TERMINOLOGY • Nuclear enlargement > 3x size (area) of intermediate cell • Squamous cell changes associated with HPV infection nucleus with mild increase in nucleus:cytoplasm ratio • Includes koilocytosis, mild dysplasia/cervical intraepithelial • Variable hyperchromasia, size, shape, and number lesion grade 1 (CIN 1) (binucleated and multinucleated) • Coarsely granular and uniformly distributed or densely ETIOLOGY/PATHOGENESIS opaque and smudged • Most low-grade squamous intraepithelial lesions (LSILs) are • Nuclear contours are smooth or slightly irregular due to high-risk HPV (HR-HPV) types (85% per ASC-US Low- • Sharply delineated perinuclear clearing with peripheral rim Grade Triage Study); others due to low-risk types 6 and 11 of densely stained cytoplasm (koilocyte) is characteristic but Gynecologic Cytopathology: • HPV-16 dominates in HR-HPV(+) group not requirement for diagnosis CLINICAL ISSUES • Cytoplasm may be densely keratinized (orangeophilic) • Perinuclear halos in absence of nuclear abnormalities do • Asymptomatic; presents as abnormal Pap smear/test not qualify for a diagnosis of LSIL • Often regresses spontaneously over period of 1-2 years • Terminology for cytology and histology unified after lower • Persistence is indicator for coexistent high-grade squamous Squamous Cell Abnormalities and Mimics anogenital squamous terminology conference in 2012 intraepithelial lesion (HSIL), which is biologically • Interpretive traps include navicular cells, radiation changes, independent event early herpes viral changes, tight halos of reactive changes CYTOPATHOLOGY • Mature cell types (i.e., superficial or intermediate) LSIL, Koilocytes on ThinPrep LSIL (Left) This ThinPrep Pap test demonstrates a group of uni- and bi-nuclear koilocytes with an optically clear halo and nuclear enlargement, hyperchromasia, and contour irregularities. -

Role and Predictive Strength of Transglutaminase Type 2 Expression in Premalignant Lesions of the Cervix
Modern Pathology (2011) 24, 855–865 & 2011 USCAP, Inc. All rights reserved 0893-3952/11 $32.00 855 Role and predictive strength of transglutaminase type 2 expression in premalignant lesions of the cervix Franca Del Nonno1, Giuseppe Pisani2, Paolo Visca3, Fabrizio Signore2, Lucia Rosalba Grillo4, Andrea Baiocchini1, Anna Rosa Garbuglia5,SaraSepe6,7, Mauro Piacentini7,8 and Laura Falasca7 1Department of Pathology, INMI-IRCCS ‘Lazzaro Spallanzani’, Rome, Italy; 2Department of Obstetrics and Gynaecology, ‘San Camillo-Forlanini’ Hospital, Rome, Italy; 3Depatment of Pathology, Regina Elena Institute, Rome, Italy; 4Department of Pathology, ‘San Camillo-Forlanini’ Hospital, Rome, Italy; 5Laboratory of Virology, INMI-IRCCS ‘Lazzaro Spallanzani’, Rome, Italy; 6Department of Biology-LIME, University ‘Roma Tre’, Rome, Italy; 7Lab of Electron Microscopy, National Institute of Infectious Diseases, INMI-IRCCS ‘Lazzaro Spallanzani’, Rome, Italy and 8Department of Biology, University of Rome ‘Tor Vergata’, Rome, Italy The demonstration that type 2 transglutaminase (TG2) can incorporate polyamine into the E7 oncoprotein of human papillomavirus (HPV) type 18 has led to the hypothesis that TG2 can have a role in the host cellular response to HPV infection. The aim of this study was to investigate whether HPV-related pathology, in infected human cervical epithelium, was associated with modulation of TG2 expression. Normal controls and HPV- infected cervical biopsies were analyzed for the expression of TG2, and the findings were compared with lesion grade. The correlation between TG2 expression and p16, a marker for HPV-induced dysplasia, and the retinoblastoma protein (Rb), a target of the E7 protein of HPV, was also investigated. Results obtained showed that TG2 was absent in normal squamous mucosa, whereas it was present in 100% CIN I lesions. -

The Evolving Management of LSIL in Pap Tests
Cytopathology in Focus: The evolving management of LSIL in Pap tests Stacey Barron Miller, MD Chengquan Zhao, MD August 2016—The Bethesda System for Reporting Cervical/Vaginal Cytologic Diagnoses was developed to establish standardized terminology among pathologists for communicating to clinicians the findings of a Pap test.1 The Bethesda System has also facilitated the examination of the epidemiology and pathogenesis of cervical disease, with a focus on low- grade and high-grade squamous intraepithelial lesions (LSIL and HSIL, respectively) and their relationships to human papillomavirus infection and progression to invasive cervical carcinoma. This accumulating knowledge has allowed for the development of cervical cancer screening algorithms and management guidelines set forth by the American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology. These algorithms and guidelines are subject to modification as knowledge of cervical disease advances and data on risk of disease accumulate. It is universally accepted at this time that women with cytologic findings of HSIL require colposcopy or surgical excision given the high risk of identifying a CIN2+ lesion on histologic examination. The clinical management of LSIL, however, has continued to evolve with the changing screening recommendations, namely HPV co-testing and longer screening intervals. Fig. 1. One typical koilocyte (600×) with peri-nuclear cavitation and nuclear enlargement. LSIL is a category recognized in the Bethesda System that is characterized by the cytologic features of HPV infection. Classically, LSIL is a lesion of intermediate and superficial squamous cells with enlarged, hyperchromatic nuclei with irregular nuclear contours and perinuclear cytoplasmic clearing, referred to as koilocytic change.1 Figs.