Howcan Heat Illness Be Affected by Medications?
Total Page:16
File Type:pdf, Size:1020Kb
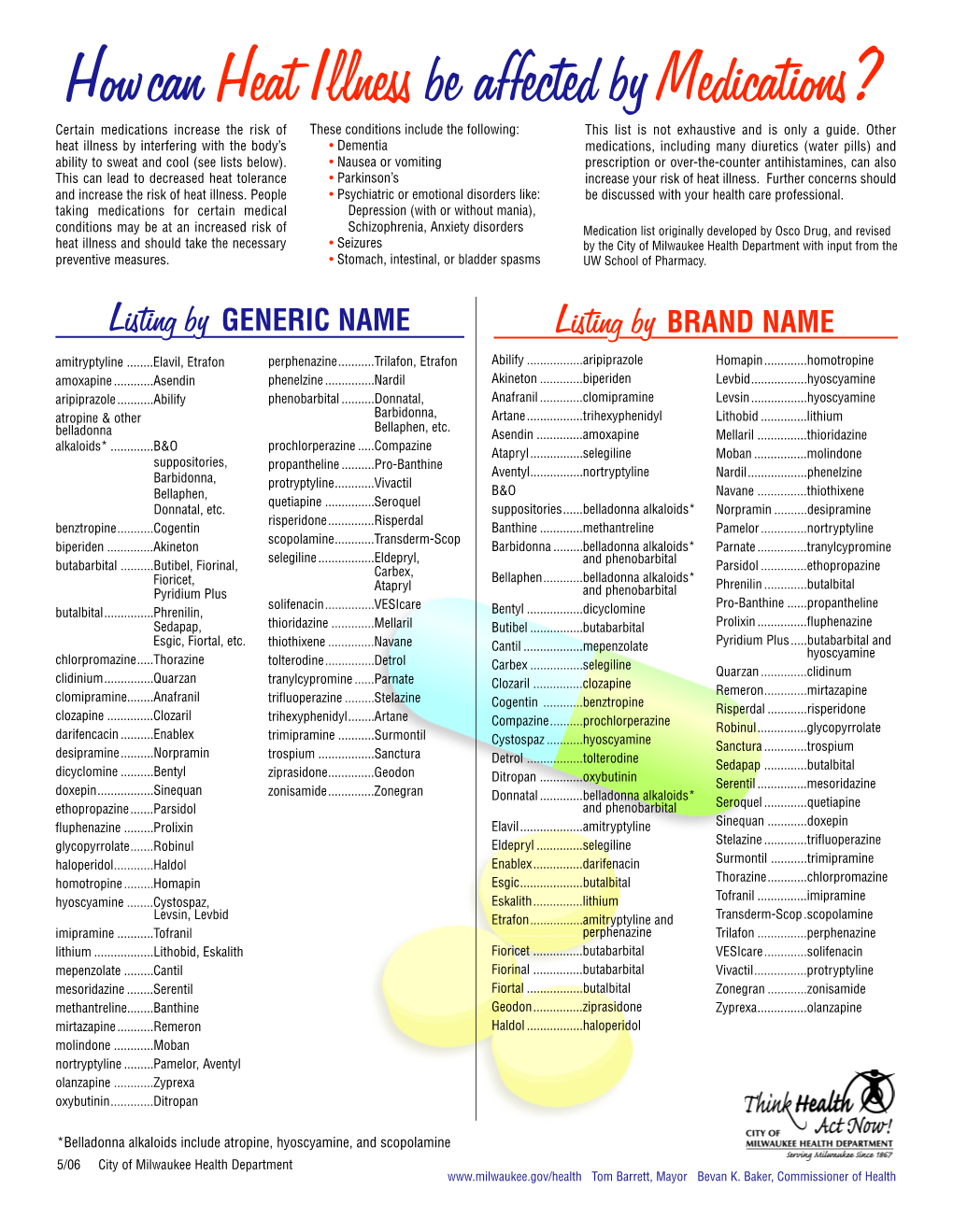
Load more
Recommended publications
-

Table 2. 2012 AGS Beers Criteria for Potentially
Table 2. 2012 AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults Strength of Organ System/ Recommendat Quality of Recomm Therapeutic Category/Drug(s) Rationale ion Evidence endation References Anticholinergics (excludes TCAs) First-generation antihistamines Highly anticholinergic; Avoid Hydroxyzin Strong Agostini 2001 (as single agent or as part of clearance reduced with e and Boustani 2007 combination products) advanced age, and promethazi Guaiana 2010 Brompheniramine tolerance develops ne: high; Han 2001 Carbinoxamine when used as hypnotic; All others: Rudolph 2008 Chlorpheniramine increased risk of moderate Clemastine confusion, dry mouth, Cyproheptadine constipation, and other Dexbrompheniramine anticholinergic Dexchlorpheniramine effects/toxicity. Diphenhydramine (oral) Doxylamine Use of diphenhydramine in Hydroxyzine special situations such Promethazine as acute treatment of Triprolidine severe allergic reaction may be appropriate. Antiparkinson agents Not recommended for Avoid Moderate Strong Rudolph 2008 Benztropine (oral) prevention of Trihexyphenidyl extrapyramidal symptoms with antipsychotics; more effective agents available for treatment of Parkinson disease. Antispasmodics Highly anticholinergic, Avoid Moderate Strong Lechevallier- Belladonna alkaloids uncertain except in Michel 2005 Clidinium-chlordiazepoxide effectiveness. short-term Rudolph 2008 Dicyclomine palliative Hyoscyamine care to Propantheline decrease Scopolamine oral secretions. Antithrombotics Dipyridamole, oral short-acting* May -

Prochlorperazine 5Mg Tablets
Package leaflet: Information for the patient Prochlorperazine 5mg tablets Read all of this leaflet carefully before you start taking this • the person is a child. This is because children may develop unusual face and body medicine, because it contains important information for you. movements (dystonic reactions) • Keep this leaflet. You may need to read it again. • you are diabetic or have high levels of sugar in your blood (hyperglycaemia). Your doctor • If you have any further questions, ask your doctor or pharmacist. may want to monitor you more closely. • This medicine has been prescribed for you only. Do not pass it on to If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before others. It may harm them, even if their signs of illness are the same taking Prochlorperazine Tablets. as yours. Other medicines and Prochlorperazine tablets • If you get any side effects, talk to your doctor or pharmacist. Tell your doctor or pharmacist if you are taking, have recently taken or might take any This includes any possible side effects not listed in this leaflet. other medicines. This includes medicines you buy without a prescription, including herbal See section 4. medicines. This is because Prochlorperazine Tablets can affect the way some other medicines work. What is in this leaflet: Also some medicines can affect the way Prochlorperazine Tablets work. 1 What Prochlorperazine tablets are and what they In particular, tell your doctor if you are taking any of the following: • medicines to help you sleep -

HIGHLIGHTS of PRESCRIBING INFORMATION These Highlights Do
HIGHLIGHTS OF PRESCRIBING INFORMATION • Metabolic Changes: Atypical antipsychotic drugs have been associated with These highlights do not include all the information needed to use metabolic changes that may increase cardiovascular/cerebrovascular risk. CLOZARIL safely and effectively. See full prescribing information for These metabolic changes include: CLOZARIL. o Hyperglycemia and Diabetes Mellitus: Monitor for symptoms of CLOZARIL® (clozapine) tablets, for oral use hyperglycemia including polydipsia, polyuria, polyphagia, and Initial U.S. Approval: 1989 weakness. Monitor glucose regularly in patients with diabetes or at risk for diabetes. (5.9) WARNING: SEVERE NEUTROPENIA; ORTHOSTATIC o Dyslipidemia: Undesirable alterations in lipids have occurred in HYPOTENSION, BRADYCARDIA, AND SYNCOPE; SEIZURE; patients treated with atypical antipsychotics. (5.9) MYOCARDITIS AND CARDIOMYOPATHY; INCREASED o Weight Gain: Significant weight gain has occurred. Monitor weight MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA- gain. (5.9) RELATED PSYCHOSIS • Neuroleptic Malignant Syndrome (NMS): Immediately discontinue and See full prescribing information for complete boxed warning. monitor closely. Assess for co-morbid conditions. (5.10) • Fever: Evaluate for infection and for neutropenia, NMS. (5.11) • Pulmonary Embolism (PE): Consider PE if respiratory distress, chest pain, • Severe Neutropenia: CLOZARIL can cause severe neutropenia, which or deep-vein thrombosis occur. (5.12) can lead to serious and fatal infections. Patients initiating and • Anticholinergic Toxicity: Use cautiously in presence of specific conditions continuing treatment with CLOZARIL must have a baseline blood (e.g., narrow angle glaucoma, use of anticholinergic drugs). (5.13) absolute neutrophil count (ANC) measured before treatment initiation • Interference with Cognitive and Motor Performance: Advise caution when and regular ANC monitoring during treatment (2.1, 5.1). -
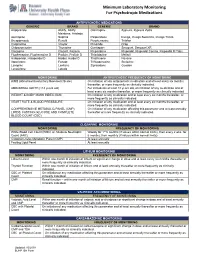
Minimum Laboratory Monitoring for Psychotropic Medications
Minimum Laboratory Monitoring For Psychotropic Medications ANTIPSYCHOTIC MEDICATIONS GENERIC BRAND GENERIC BRAND Aripiprazole Abilify, Abilify Olanzapine Zyprexa, Zyprexa Zydis Maintena, Aristada Asenapine Saphris Paliperidone Invega, Invega Sustenna, Invega Trinza Brexpiprazole Rexulti Perphenazine Trilafon Cariprazine Vraylar Pimozide Orap Chlorpromazine Thorazine Quetiapine Seroquel, Seroquel XR Clozapine Clozaril, Fazaclo Risperidone Risperdal, Risperdal Consta, Risperdal M Tabs Fluphenazine, Fluphenazine D Prolixin, Prolixin D Thioridazine Mellaril Haloperidol, Haloperidol D Haldol, Haldol D Thiothixene Navane Iloperidone Fanapt Trifluoperazine Stelazine Loxapine Loxitane Ziprasidone Geodon Lurasidone Latuda MONITORING ANTIPSYCHOTIC FREQUENCY OF MONITORING AIMS (Abnormal Involuntary Movement Scale) On initiation of any antipsychotic medication and at least every six months thereafter, or more frequently as clinically indicated. ABDOMINAL GIRTH (>18 years old) For individuals at least 18 years old, on initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. WEIGHT & BODY MASS INDEX (BMI) On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. HEART RATE & BLOOD PRESSSURE On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. COMPREHENSIVE METABOLIC PANEL (CMP), On initiation of any medication affecting this parameter and at least annually LIPIDS, FASTING -

Anticholinergic Drugs Improve Symptoms but Increase Dry Mouth in Adults with Overactive Bladder Syndrome
Source of funding: Evid Based Nurs: first published as 10.1136/ebn.6.2.49 on 1 April 2003. Downloaded from Review: anticholinergic drugs improve symptoms but Health Research Council of Aotearoa increase dry mouth in adults with overactive bladder New Zealand. syndrome For correspondence: Jean Hay-Smith, Hay-Smith J, Herbison P,Ellis G, et al. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Dunedin School of Cochrane Database Syst Rev 2002;(3):CD003781 (latest version May 29 2002). Medicine, University of Otago, Dunedin, New QUESTION: What are the effects of anticholinergic drugs in adults with overactive Zealand. jean.hay-smith@ bladder syndrome? otago.ac.nz Data sources Parallel arm studies of anticholinergic drugs v placebo for overactive bladder syndrome in Studies were identified by searching the Cochrane adults at 12 days to 12 weeks* Incontinence Group trials register (to January 2002) Weighted event rates and reference lists of relevant papers. Anticholinergic Study selection Outcomes drugs Placebo RBI (95% CI) NNT (CI) Randomised or quasi-randomised controlled trials in Self reported cure or adults with symptomatic diagnosis of overactive bladder improvement (8 studies) 63% 45% 41% (29 to 54) 6 (5 to 8) syndrome, urodynamic diagnosis of detrusor overactiv- RRI (CI) NNH (CI) ity, or both, that compared an anticholinergic drug Dry mouth (20 studies) 36% 15% 138 (70 to 232) 5 (4 to 7) (given to decrease symptoms of overactive bladder) with Outcomes Weighted mean difference (CI) placebo or no treatment. Studies of darifenacin, emepronium bromide or carrageenate, dicyclomine Number of leakage episodes in 24 hours (9 − chloride, oxybutynin chloride, propiverine, propanthe- studies) 0.56 (–0.73 to –0.39) Number of micturitions in 24 hours (8 studies) −0.59 (–0.83 to –0.36) line bromide, tolterodine, and trospium chloride were Maximum cystometric volume (ml) (12 studies) 54.3 (43.0 to 65.7) included. -

Medication Conversion Chart
Fluphenazine FREQUENCY CONVERSION RATIO ROUTE USUAL DOSE (Range) (Range) OTHER INFORMATION KINETICS Prolixin® PO to IM Oral PO 2.5-20 mg/dy QD - QID NA ↑ dose by 2.5mg/dy Q week. After symptoms controlled, slowly ↓ dose to 1-5mg/dy (dosed QD) Onset: ≤ 1hr 1mg (2-60 mg/dy) Caution for doses > 20mg/dy (↑ risk EPS) Cmax: 0.5hr 2.5mg Elderly: Initial dose = 1 - 2.5mg/dy t½: 14.7-15.3hr 5mg Oral Soln: Dilute in 2oz water, tomato or fruit juice, milk, or uncaffeinated carbonated drinks Duration of Action: 6-8hr 10mg Avoid caffeinated drinks (coffee, cola), tannics (tea), or pectinates (apple juice) 2° possible incompatibilityElimination: Hepatic to inactive metabolites 5mg/ml soln Hemodialysis: Not dialyzable HCl IM 2.5-10 mg/dy Q6-8 hr 1/3-1/2 po dose = IM dose Initial dose (usual): 1.25mg Onset: ≤ 1hr Immediate Caution for doses > 10mg/dy Cmax: 1.5-2hr Release t½: 14.7-15.3hr 2.5mg/ml Duration Action: 6-8hr Elimination: Hepatic to inactive metabolites Hemodialysis: Not dialyzable Decanoate IM 12.5-50mg Q2-3 wks 10mg po = 12.5mg IM CONVERTING FROM PO TO LONG-ACTING DECANOATE: Onset: 24-72hr (4-72hr) Long-Acting SC (12.5-100mg) (1-4 wks) Round to nearest 12.5mg Method 1: 1.25 X po daily dose = equiv decanoate dose; admin Q2-3wks. Cont ½ po daily dose X 1st few mths Cmax: 48-96hr 25mg/ml Method 2: ↑ decanoate dose over 4wks & ↓ po dose over 4-8wks as follows (accelerate taper for sx of EPS): t½: 6.8-9.6dy (single dose) ORAL DECANOATE (Administer Q 2 weeks) 15dy (14-100dy chronic administration) ORAL DOSE (mg/dy) ↓ DOSE OVER (wks) INITIAL DOSE (mg) TARGET DOSE (mg) DOSE OVER (wks) Steady State: 2mth (1.5-3mth) 5 4 6.25 6.25 0 Duration Action: 2wk (1-6wk) Elimination: Hepatic to inactive metabolites 10 4 6.25 12.5 4 Hemodialysis: Not dialyzable 20 8 6.25 12.5 4 30 8 6.25 25 4 40 8 6.25 25 4 Method 3: Admin equivalent decanoate dose Q2-3wks. -

Schizophrenia Care Guide
August 2015 CCHCS/DHCS Care Guide: Schizophrenia SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT GOALS ALERTS Minimize frequency and severity of psychotic episodes Suicidal ideation or gestures Encourage medication adherence Abnormal movements Manage medication side effects Delusions Monitor as clinically appropriate Neuroleptic Malignant Syndrome Danger to self or others DIAGNOSTIC CRITERIA/EVALUATION (PER DSM V) 1. Rule out delirium or other medical illnesses mimicking schizophrenia (see page 5), medications or drugs of abuse causing psychosis (see page 6), other mental illness causes of psychosis, e.g., Bipolar Mania or Depression, Major Depression, PTSD, borderline personality disorder (see page 4). Ideas in patients (even odd ideas) that we disagree with can be learned and are therefore not necessarily signs of schizophrenia. Schizophrenia is a world-wide phenomenon that can occur in cultures with widely differing ideas. 2. Diagnosis is made based on the following: (Criteria A and B must be met) A. Two of the following symptoms/signs must be present over much of at least one month (unless treated), with a significant impact on social or occupational functioning, over at least a 6-month period of time: Delusions, Hallucinations, Disorganized Speech, Negative symptoms (social withdrawal, poverty of thought, etc.), severely disorganized or catatonic behavior. B. At least one of the symptoms/signs should be Delusions, Hallucinations, or Disorganized Speech. TREATMENT OPTIONS MEDICATIONS Informed consent for psychotropic -

Management of Side Effects of Antipsychotics
Management of side effects of antipsychotics Oliver Freudenreich, MD, FACLP Co-Director, MGH Schizophrenia Program www.mghcme.org Disclosures I have the following relevant financial relationship with a commercial interest to disclose (recipient SELF; content SCHIZOPHRENIA): • Alkermes – Consultant honoraria (Advisory Board) • Avanir – Research grant (to institution) • Janssen – Research grant (to institution), consultant honoraria (Advisory Board) • Neurocrine – Consultant honoraria (Advisory Board) • Novartis – Consultant honoraria • Otsuka – Research grant (to institution) • Roche – Consultant honoraria • Saladax – Research grant (to institution) • Elsevier – Honoraria (medical editing) • Global Medical Education – Honoraria (CME speaker and content developer) • Medscape – Honoraria (CME speaker) • Wolters-Kluwer – Royalties (content developer) • UpToDate – Royalties, honoraria (content developer and editor) • American Psychiatric Association – Consultant honoraria (SMI Adviser) www.mghcme.org Outline • Antipsychotic side effect summary • Critical side effect management – NMS – Cardiac side effects – Gastrointestinal side effects – Clozapine black box warnings • Routine side effect management – Metabolic side effects – Motor side effects – Prolactin elevation • The man-in-the-arena algorithm www.mghcme.org Receptor profile and side effects • Alpha-1 – Hypotension: slow titration • Dopamine-2 – Dystonia: prophylactic anticholinergic – Akathisia, parkinsonism, tardive dyskinesia – Hyperprolactinemia • Histamine-1 – Sedation – Weight gain -

Drugs to Avoid in Patients with Dementia
Detail-Document #240510 -This Detail-Document accompanies the related article published in- PHARMACIST’S LETTER / PRESCRIBER’S LETTER May 2008 ~ Volume 24 ~ Number 240510 Drugs To Avoid in Patients with Dementia Elderly people with dementia often tolerate drugs less favorably than healthy older adults. Reasons include increased sensitivity to certain side effects, difficulty with adhering to drug regimens, and decreased ability to recognize and report adverse events. Elderly adults with dementia are also more prone than healthy older persons to develop drug-induced cognitive impairment.1 Medications with strong anticholinergic (AC) side effects, such as sedating antihistamines, are well- known for causing acute cognitive impairment in people with dementia.1-3 Anticholinergic-like effects, such as urinary retention and dry mouth, have also been identified in drugs not typically associated with major AC side effects (e.g., narcotics, benzodiazepines).3 These drugs are also important causes of acute confusional states. Factors that may determine whether a patient will develop cognitive impairment when exposed to ACs include: 1) total AC load (determined by number of AC drugs and dose of agents utilized), 2) baseline cognitive function, and 3) individual patient pharmacodynamic and pharmacokinetic features (e.g., renal/hepatic function).1 Evidence suggests that impairment of cholinergic transmission plays a key role in the development of Alzheimer’s dementia. Thus, the development of the cholinesterase inhibitors (CIs). When used appropriately, the CIs (donepezil [Aricept], rivastigmine [Exelon], and galantamine [Razadyne, Reminyl in Canada]) may slow the decline of cognitive and functional impairment in people with dementia. In order to achieve maximum therapeutic effect, they ideally should not be used in combination with ACs, agents known to have an opposing mechanism of action.1,2 Roe et al studied AC use in 836 elderly patients.1 Use of ACs was found to be greater in patients with probable dementia than healthy older adults (33% vs. -

The Effects of Antipsychotic Treatment on Metabolic Function: a Systematic Review and Network Meta-Analysis
The effects of antipsychotic treatment on metabolic function: a systematic review and network meta-analysis Toby Pillinger, Robert McCutcheon, Luke Vano, Katherine Beck, Guy Hindley, Atheeshaan Arumuham, Yuya Mizuno, Sridhar Natesan, Orestis Efthimiou, Andrea Cipriani, Oliver Howes ****PROTOCOL**** Review questions 1. What is the magnitude of metabolic dysregulation (defined as alterations in fasting glucose, total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, and triglyceride levels) and alterations in body weight and body mass index associated with short-term (‘acute’) antipsychotic treatment in individuals with schizophrenia? 2. Does baseline physiology (e.g. body weight) and demographics (e.g. age) of patients predict magnitude of antipsychotic-associated metabolic dysregulation? 3. Are alterations in metabolic parameters over time associated with alterations in degree of psychopathology? 1 Searches We plan to search EMBASE, PsycINFO, and MEDLINE from inception using the following terms: 1 (Acepromazine or Acetophenazine or Amisulpride or Aripiprazole or Asenapine or Benperidol or Blonanserin or Bromperidol or Butaperazine or Carpipramine or Chlorproethazine or Chlorpromazine or Chlorprothixene or Clocapramine or Clopenthixol or Clopentixol or Clothiapine or Clotiapine or Clozapine or Cyamemazine or Cyamepromazine or Dixyrazine or Droperidol or Fluanisone or Flupehenazine or Flupenthixol or Flupentixol or Fluphenazine or Fluspirilen or Fluspirilene or Haloperidol or Iloperidone -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

Management of Major Depressive Disorder Clinical Practice Guidelines May 2014
Federal Bureau of Prisons Management of Major Depressive Disorder Clinical Practice Guidelines May 2014 Table of Contents 1. Purpose ............................................................................................................................................. 1 2. Introduction ...................................................................................................................................... 1 Natural History ................................................................................................................................. 2 Special Considerations ...................................................................................................................... 2 3. Screening ........................................................................................................................................... 3 Screening Questions .......................................................................................................................... 3 Further Screening Methods................................................................................................................ 4 4. Diagnosis ........................................................................................................................................... 4 Depression: Three Levels of Severity ............................................................................................... 4 Clinical Interview and Documentation of Risk Assessment...............................................................