Effects and Use of Some Ocimum Plant Species and Their Essential Oils on Some Storage Insect Pests
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Working List of Prairie Restricted (Specialist) Insects in Wisconsin (11/26/2015)
Working List of Prairie Restricted (Specialist) Insects in Wisconsin (11/26/2015) By Richard Henderson Research Ecologist, WI DNR Bureau of Science Services Summary This is a preliminary list of insects that are either well known, or likely, to be closely associated with Wisconsin’s original native prairie. These species are mostly dependent upon remnants of original prairie, or plantings/restorations of prairie where their hosts have been re-established (see discussion below), and thus are rarely found outside of these settings. The list also includes some species tied to native ecosystems that grade into prairie, such as savannas, sand barrens, fens, sedge meadow, and shallow marsh. The list is annotated with known host(s) of each insect, and the likelihood of its presence in the state (see key at end of list for specifics). This working list is a byproduct of a prairie invertebrate study I coordinated from1995-2005 that covered 6 Midwestern states and included 14 cooperators. The project surveyed insects on prairie remnants and investigated the effects of fire on those insects. It was funded in part by a series of grants from the US Fish and Wildlife Service. So far, the list has 475 species. However, this is a partial list at best, representing approximately only ¼ of the prairie-specialist insects likely present in the region (see discussion below). Significant input to this list is needed, as there are major taxa groups missing or greatly under represented. Such absence is not necessarily due to few or no prairie-specialists in those groups, but due more to lack of knowledge about life histories (at least published knowledge), unsettled taxonomy, and lack of taxonomic specialists currently working in those groups. -

'Ofe Akwu' Soup Spoilage
Microbiology Research Journal International 19(3): 1-8, 2017; Article no.MRJI.32554 Previously known as British Microbiology Research Journal ISSN: 2231-0886, NLM ID: 101608140 SCIENCEDOMAIN international www.sciencedomain.org Inhibitory Potential of Ocimum gratissimum L on Bacterial Implicated in ‘Ofe Akwu’ Soup Spoilage O. C. Eruteya 1* , F. S. Ire 1 and C. C. Aneke 1 1Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria. Authors’ contributions This work was carried out in collaboration between all authors. Authors OCE and FSI designed the study. All authors participated in the laboratory analysis. Author OCE wrote the first draft of the manuscript. All authors read and approved the final manuscript. Article Information DOI: 10.9734/MRJI/2017/32554 Editor(s): (1) Ana Cláudia Coelho, Department of Veterinary Sciences, University of Trás-os-Montes and Alto Douro, Portugal. Reviewers: (1) Vishwanadham Yerragunta, JNTU-Hydrabad, India. (2) P. Rameshthangam, Alagappa University, Karaikudi, Tamilnadu, India. (3) Julius Tibyangye, St. Augustine International University/Kampala International University, Uganda. Complete Peer review History: http://www.sciencedomain.org/review-history/18590 Received 1st March 2017 Accepted 29 th March 2017 Original Research Article th Published 11 April 2017 ABSTRACT Aims: The study evaluated the proximate composition, the bacteria present in freshly spoilt ‘ofe akwu’ soup and inhibitory potential of crude ethanol, methanol and aqueous leaf extract of Ocimum gratissimum on the resulting bacteria. Study Design: This was an analytical study in duplicate. Place and Duration of Study: Department of Microbiology, University of Port Harcourt, Niger Delta University, Amasoma, and South Africa, between July 2015 and December 2016. -
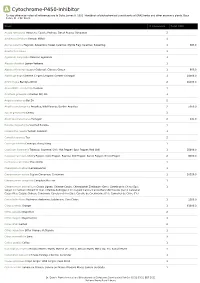
Show Activity
A Cytochrome-P450-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Acacia farnesiana Huisache; Cassie; Popinac; Sweet Acacia; Opopanax 2 Achillea millefolium Yarrow; Milfoil 1 Acorus calamus Flagroot; Sweetroot; Sweet Calamus; Myrtle Flag; Calamus; Sweetflag 1 384.0 Agastache rugosa 1 Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia officinarum Lesser Galangal; Chinese Ginger 1 800.0 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 24000.0 Ammi majus Bishop's Weed 2 16000.0 Anacardium occidentale Cashew 1 Anethum graveolens Garden Dill; Dill 1 Angelica dahurica Bai Zhi 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 5050.0 Apium graveolens Celery 3 Artemisia dracunculus Tarragon 2 141.0 Boronia megastigma Scented Boronia 1 Calamintha nepeta Turkish Calamint 1 Camellia sinensis Tea 2 Cananga odorata Cananga; Ylang-Ylang 1 Capsicum frutescens Tabasco; Cayenne; Chili; Hot Pepper; Spur Pepper; Red Chili 1 35800.0 Capsicum annuum Cherry Pepper; Cone Pepper; Paprika; Bell Pepper; Sweet Pepper; Green Pepper 2 8000.0 Centaurea calcitrapa Star-Thistle 1 Chenopodium album Lambsquarter 1 Cinnamomum verum Ceylon Cinnamon; Cinnamon 1 20320.0 Cinnamomum camphora Camphor; Ho Leaf 1 Cinnamomum aromaticum Cassia Lignea; Chinese Cassia; Chinesischer Zimtbaum (Ger.); Canela de la China (Sp.); 1 Saigon Cinnamon; Chinazimt (Ger.); Kashia-Keihi -

Seasonal Incidence of Ocimum Tingid Bug, Cochlochila Bullita Stal
Journal of Pharmacognosy and Phytochemistry 2020; 9(4): 3138-3144 E-ISSN: 2278-4136 P-ISSN: 2349-8234 www.phytojournal.com Seasonal incidence of Ocimum tingid bug, JPP 2020; 9(4): 3138-3144 Received: 07-05-2020 Cochlochila bullita Stal (Heteroptera: Tingidae) Accepted: 09-06-2020 on three different species of Ocimum viz. Ocimum Vishav Prakash Rahul basilicum L., Ocimum sanctum L. and Ocimum CSIR-Indian Institute of Integrative Medicine, Canal kilimandscharicum Guerke Road, Jammu, Jammu and Kashmir, India Vishav Prakash Rahul, Bhumika Kapoor, Sougata Sarkar and Sabha Jeet Bhumika Kapoor Project Assistant, CSIR-Indian Institute of Integrative DOI: https://doi.org/10.22271/phyto.2020.v9.i4ae.12093 Medicine, Canal Road, Jammu, Jammu and Kashmir, India Abstract The field experiment was conducted on the seasonal incidence Ocimum lace bug on three different Sougata Sarkar species of Ocimum viz. Ocimum basilicum L., Ocimum sanctum L. and Ocimum kilimandscharicum Research Associate, Scientist, Guerke at CSIR- IIIM, Chatha Farm, Jammu. The data on seasonal fluctuations of Ocimum tingid bug, CSIR-Indian Institute of Cochlochila bullita on various species of Ocimum such as Ocimum basilicum L., Ocimum sanctum L. Integrative Medicine, Canal and Ocimum kilimandscharicum Guerke were first observed during 33rd standard week of August i.e. Road, Jammu, Jammu and 0.40 Mean insect/ plant, 0.2 mean insect/plant and 0.20 mean insect/plant, respectively. The maximum Kashmir, India lace bug population was recorded on sweet basil during 39th standard week i.e. 52.60 mean insect/ plant when weekly mean maximum temperature 29.7 oC, minimum temperature 23.1 oC, morning relative Sabha Jeet Scientist, CSIR-Indian Institute humidity 93.1% and evening relative humidity 75.90 %, rainfall 93.40 mm, and wind speed 2.00 km/hr, of Integrative Medicine, Canal respectively. -

Volume 42, Number 2 June 2015
Wisconsin Entomological Society N e w s I e t t e r Volume 42, Number 2 June 2015 Monitoring and Management - A That is, until volunteer moth surveyor, Steve Sensible Pairing Bransky, came onto the scene. Steve had By Beth Goeppinger, Wisconsin Department done a few moth and butterfly surveys here ofN atural Resources and there on the property. But that changed in 2013. Armed with mercury vapor lights, Richard Bong State Recreation Area is a bait and a Wisconsin scientific collector's heavily used 4,515 acre property in the permit, along with our permission, he began Wisconsin State Park system. It is located in surveying in earnest. western Kenosha County. The area is oak woodland, savanna, wetland, sedge meadow, He chose five sites in woodland, prairie and old field and restored and remnant prairie. savanna habitats. He came out many nights Surveys of many kinds and for many species in the months moths might be flying. After are done on the property-frog and toad, finding that moth populations seemed to drift fence, phenology, plants, ephemeral cycle every 3-5 days, he came out more ponds, upland sandpiper, black tern, frequently. His enthusiasm, dedication and grassland and marsh birds, butterfly, small never-ending energy have wielded some mammal, waterfowl, muskrat and wood surprising results. Those results, in turn, ducks to name a few. Moths, except for the have guided us in our habitat management showy and easy-to-identify species, have practices. been ignored. Of the 4,500 moth species found in the state, Steve has confirmed close to 1,200 on the property, and he isn't done yet! He found one of the biggest populations of the endangered Papaipema silphii moths (Silphium borer) in the state as well as 36 species of Catocola moths (underwings), them. -

Serpentine Geoecology of Eastern North America: a Review
RHODORA, Vol. 111, No. 945, pp. 21–108, 2009 E Copyright 2009 by the New England Botanical Club SERPENTINE GEOECOLOGY OF EASTERN NORTH AMERICA: A REVIEW NISHANTA RAJAKARUNA College of the Atlantic, 105 Eden Street, Bar Harbor, ME 04609 Current Address: Department of Biological Sciences, One Washington Square, San Jose´ State University, San Jose´, CA 95192-0100 e-mail: [email protected] TANNER B. HARRIS University of Massachusetts, Fernald Hall, 270 Stockbridge Road, Amherst, MA 01003 EARL B. ALEXANDER 1714 Kasba Street, Concord, CA 94518 ABSTRACT. Serpentine outcrops are model habitats for geoecological studies. While much attention has been paid to serpentine outcrops worldwide, the literature on eastern North American serpentine and associated biota is scant. This review examines the available literature, published and unpublished, on geoecological studies conducted on serpentine in eastern North America, from Newfoundland through Que´bec and New England south to Alabama. Most serpentine outcrops in the region have been mapped, but there have been few intensive mineralogical and pedological investigations. The limited soil analyses available suggest elevated levels of heavy metals such as Ni, near-neutralpH values, and Ca:Mg ratios , 1, characteristic of serpentine soils worldwide. Botanical studies to date have largely focused on floristic surveys and the influence of fire exclusion and grazing on indigenous vegetation. To date, 751 taxa of vascular plants belonging to 92 families have been reported from serpentine outcrops in the region. Two taxa, Agalinis acuta and Schwalbea americana, are federally endangered in the United States while many others are listed as rare, endangered, or imperiled in one or more states or provinces. -

Honey Bee Suite © Rusty Burlew 2015 Master Plant List by Scientific Name United States
Honey Bee Suite Master Plant List by Scientific Name United States © Rusty Burlew 2015 Scientific name Common Name Type of plant Zone Full Link for more information Abelia grandiflora Glossy abelia Shrub 6-9 http://plants.ces.ncsu.edu/plants/all/abelia-x-grandiflora/ Acacia Acacia Thorntree Tree 3-8 http://www.2020site.org/trees/acacia.html Acer circinatum Vine maple Tree 7-8 http://www.nwplants.com/business/catalog/ace_cir.html Acer macrophyllum Bigleaf maple Tree 5-9 http://treesandshrubs.about.com/od/commontrees/p/Big-Leaf-Maple-Acer-macrophyllum.htm Acer negundo L. Box elder Tree 2-10 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=a841 Acer rubrum Red maple Tree 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=275374&isprofile=1&basic=Acer%20rubrum Acer rubrum Swamp maple Tree 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=275374&isprofile=1&basic=Acer%20rubrum Acer saccharinum Silver maple Tree 3-9 http://en.wikipedia.org/wiki/Acer_saccharinum Acer spp. Maple Tree 3-8 http://en.wikipedia.org/wiki/Maple Achillea millefolium Yarrow Perennial 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=b282 Aesclepias tuberosa Butterfly weed Perennial 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=b490 Aesculus glabra Buckeye Tree 3-7 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=281045&isprofile=1&basic=buckeye -

Herb List Gardens
NORTH HAVEN Herb List Gardens Common Name Botanical Name UsesCat Light Color Height Soil Symbolism ALOE VERA Aloe barbadensis M TT SUNOrangeWD12"-18" Healing ANISE Pimpinella anisum CMTA S/PSH White12"-18" MWD APPLE MINT Mentha suaveolens CFr MTP S/PSHWhite12"-18" MWD Virtue APPLE MINT Mentha rotundifolia S/PSH Mauve4"-6" M ARUGULA Eruca vesicara CM A SUNCream18"-24" MWD Enthusiasm ARUGULA, DWARF Diplotaxis erucoides C A SUNYellow10"-12" WD Straightforward BASIL, AFRICAN BLUE Ocimum kilimandscharicum CFr O A SUNPurple24"-36" M Affection BASIL, AROMA 2 Ocimum basilicum CFr Fl M O A S/PSHWhite18"-24" WD Good Luck BASIL,' AUSSIE SWEETIE' Ocimum basilicum CFr A SUN18"-24" WD Good Wishes BASIL, BOXWOOD Ocimum basilicum CFr Fl O A S/PSH White8"-10" WD BASIL, CINNAMON Ocimum basilicum CFr A SUNLavender 18"-24" MWD Good Wishes BASIL, 'CITRIODORUM' LEMON Ocimum basilicum CFr A SUNWhite18"-24" MWD Good Wishes BASIL, DARK OPAL Ocimum basilicum COA SUNPurple18"-24" MWD Good Wishes BASIL, 'GENOVESE' Ocimum basilicum CFr Fl A S/PSHWhite18"-24" MWD Good Wishes BASIL, 'GREEK COLUMNAR' OR 'AU Ocimum xcitriodorum 'Lesbos' CFr MO A S/PSH 24"-36" MWD BASIL, HOLY Ocimum sanctum Fr Fl A S/PSHWhite or Laven18"-24" MWD Good Luck BASIL, LETTUCE LEAF Ocimum basilicum COA SUNWhite18"-24" MWD Good Wishes BASIL, LIME Ocimum americanum CFr A SUNWhite18"-24" MWD Good Wishes BASIL, 'MAGICAL MICHAEL' Ocimum basilicum CFr Fl O A S/PSHWhite18"-24" WD Good Wishes BASIL, 'MINETTE' Ocimum basilicum CFr O A SUNWhite12"-18" MWD Good Wishes BASIL, MINI PURPLE Ocimum basilicum -

(Hemiptera: Heteroptera) from Wisconsin, Supplement
The Great Lakes Entomologist Volume 48 Numbers 3/4 -- Fall/Winter 2015 Numbers 3/4 -- Article 13 Fall/Winter 2015 October 2015 Feeding Records of True Bugs (Hemiptera: Heteroptera) from Wisconsin, Supplement Andrew H. Williams Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Williams, Andrew H. 2015. "Feeding Records of True Bugs (Hemiptera: Heteroptera) from Wisconsin, Supplement," The Great Lakes Entomologist, vol 48 (3) Available at: https://scholar.valpo.edu/tgle/vol48/iss3/13 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Williams: Feeding Records of True Bugs (Hemiptera: Heteroptera) from Wiscon 192 THE GREAT LAKES ENTOMOLOGIST Vol. 48, Nos. 3 - 4 Feeding Records of True Bugs (Hemiptera: Heteroptera) from Wisconsin, Supplement Andrew H. Williams Abstract In order to understand any animal and its habitat requirements, we must know what it eats. Reported here are observations of feeding by 27 species of true bugs (Hemiptera: Heteroptera) encountered in various habitats in Wisconsin over the years 2003–2014. This is the first report ofAnasa repetita Heidemann (Coreidae) from Wisconsin. ____________________ Knowing what an animal eats is essential to our understanding of that animal and its habitat requirements. Over the years 2003–2014, I accumulated many observations of insects feeding in Wisconsin. These data are vouchered by hand-collected specimens given to the Insect Research Collection of the Entomology Department at University of Wisconsin - Madison. -

Plant List 2021-08-25 (12:18)
Plant List 2021-09-24 (14:25) Plant Plant Name Botanical Name in Price Stock Per Unit AFRICAN DREAM ROOT - 1 Silene capensis Yes R92 AFRICAN DREAM ROOT - 2 Silene undulata Yes R92 AFRICAN POTATO Hypoxis hemerocallidea Yes R89 AFRICAN POTATO - SILVER-LEAFED STAR FLOWER Hypoxis rigidula Yes R89 AGASTACHE - GOLDEN JUBILEE Agastache foeniculum No R52 AGASTACHE - HYSSOP, WRINKLED GIANT HYSSOP Agastache rugosa Yes R59 AGASTACHE - LICORICE MINT HYSSOP Agastache rupestris No R59 AGASTACHE - PINK POP Agastache astromontana No R54 AGRIMONY Agrimonia eupatoria No R54 AJWAIN Trachyspermum ammi No R49 ALFALFA Medicago sativa Yes R59 ALOE VERA - ORANGE FLOWER A. barbadensis Yes R59 ALOE VERA - YELLOW FLOWER syn A. barbadensis 'Miller' No R59 AMARANTH - ‘LOVE-LIES-BLEEDING’ Amaranthus caudatus No R49 AMARANTH - CHINESE SPINACH Amaranthus species No R49 AMARANTH - GOLDEN GIANT Amaranthus cruentas No R49 AMARANTH - RED LEAF Amaranthus cruentas No R49 ARTICHOKE - GREEN GLOBE Cynara scolymus Yes R54 ARTICHOKE - JERUSALEM Helianthus tuberosus Yes R64 ARTICHOKE - PURPLE GLOBE Cynara scolymus No R54 ASHWAGANDA, INDIAN GINSENG Withania somniferia Yes R59 ASPARAGUS - GARDEN Asparagus officinalis Yes R54 BALLOON FLOWER - PURPLE Platycodon grandiflorus 'Apoyama' Yes R59 BALLOON FLOWER - WHITE Platycodon grandiflorus var. Albus No R59 BASIL - CAMPHOR Ocimum kilimandscharicum Yes R59 BASIL HOLY - GREEN TULSI, RAM TULSI Ocimum Sanctum Yes R54 BASIL HOLY - TULSI KAPOOR Ocimum sanctum Linn. No R54 BASIL HOLY - TULSI TEMPERATE Ocimum africanum No R54 BASIL HOLY - TULSI -
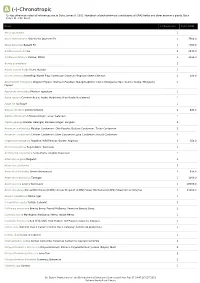
Show Activity
A (-)-Chronotropic *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Abies spectabilis 1 Abies sachalinensis Shin-Yo-Yu; Japanese Fir 1 7560.0 Abies balsamea Balsam Fir 1 4090.0 Achillea moschata Iva 2 4536.0 Achillea millefolium Yarrow; Milfoil 3 3190.0 Acinos suaveolens 2 Acinos alpinus Te de Sierra Nevada 1 Acorus calamus Sweetflag; Myrtle Flag; Sweetroot; Calamus; Flagroot; Sweet Calamus 2 200.0 Aframomum melegueta Alligator Pepper; Grains-of-Paradise; Malagettapfeffer (Ger.); Malagueta (Sp.); Guinea Grains; Melegueta 1 Pepper Ageratum conyzoides Mexican ageratum 1 Ajuga reptans Common Bugle; Bugle; Bugleherb; Blue Bugle; Bugleweed 1 Ajuga iva Ivy Bugle 1 Aloysia citrodora Lemon Verbena 2 840.0 Alpinia officinarum Chinese Ginger; Lesser Galangal 1 Alpinia galanga Greater Galangal; Siamese Ginger; Languas 3 Amomum xanthioides Malabar Cardamom; Chin Kousha; Bastard Cardamom; Tavoy Cardamom 2 Amomum compactum Chester Cardamom; Siam Cardamom; Java Cardamom; Round Cardamom 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 150.0 Annona squamosa Sugar-Apple; Sweetsop 1 Aristolochia serpentaria Serpentaria; Virginia Snakeroot 1 Artemisia vulgaris Mugwort 2 Artemisia salsoloides 3 Artemisia herba-alba Desert Wormwood 3 638.0 Artemisia dracunculus Tarragon 1 1000.0 Artemisia cina Levant Wormseed 1 48000.0 Artemisia annua Annual Wormwood (GRIN); Annual Mugwort (GRIN); Sweet Wormwood -

(12) United States Patent (10) Patent No.: US 8,808,719 B1 Flor-Weiler Et Al
US008808719B1 (12) United States Patent (10) Patent No.: US 8,808,719 B1 Flor-Weiler et al. (45) Date of Patent: Aug. 19, 2014 (54) USE OF CHROMOBACTERIUMSUBSTUGAE 3974 A. A38 Mr. FORMULATIONS, COMPOSTIONS AND 2012/010O236 A1 4/2012 AsolkarCS COMPOUNDS TO MODULATE CORNWORM ROOTWORMLARVAE INFESTATION FOREIGN PATENT DOCUMENTS (71) Applicant: Marrone Bio Innovations, Inc., Davis, KR 10-2007-00881.50 8, 2007 CA (US) WO WO91/OOO12 1, 1991 WO WOO 1/74161 10, 2001 WO WO 2004.056960 T 2004 (72) Inventors: Lina Flor-Weiler, Davis, IL (US); April WO WO 2011, 110932 9, 2011 Yang, Fremont, CA (US) WO WO 2013,062977 5, 2013 (73) Assignee: Marrone Bio Innovations, Inc., Davis, OTHER PUBLICATIONS CA (US) Martin et al., “Toxicity of Chromobacterium subtsugae to Southern - r green Stink bug (Heteroptera: Pentatomidae and corn rootworm (*) Notice: sity is titly (Coleoptera: Chrysomelidae).” J Econ Entomol 100(3):680-684. 2007.* U.S.C. 154(b) by 0 days. Asolkar et al. “Daryamides A-C. Weakly Cytotoxic Polyketides from a Marine-Derived Actinomycete of the Genus Streptomyces Strain (21) Appl. No.: 13/842.981 CNQ-085” J. Nat. Prod. 69: 1756-1759. 2006. Aspelin et al. “Pesticides Industry Sales and Usage, 1996 and 1997" (22) Filed: Mar 15, 2013 U.S EPA. Publication 733-R-99-001. 1999. Arena et al. “The Mechanism of Action of Avermectins in (51) Int. Cl. Caenorhabditis elegans: Correlation Between Activation of AOIN 25/00 (2006.01) Glutamate-Sensitive Chloride Current, Membrane Binding and Bio AOIN 63/00 (2006.01) logical Activity” Journal of Parasitology 81: 286-294.