Key Figures for 2019
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Offizielle Mitteilung Der Schweizer Boerse
Bekanntmachung auf KeyInvest Änderung der Valorennummer und ISIN der Unilever N.V. Change of the Valor number and ISIN of Unilever N.V. Im Zusammenhang mit der Änderung der Valorennummer und ISIN der Unilever N.V. (Bloomberg Code: UNA NA) wurden die Bedingungen der unten genannten Produkte der UBS AG, per 1. Juli 2019 angepasst. Die detaillierten Informationen befinden sich in der entsprechenden Produktedokumentation (Termsheets). With regard to the change of the Valor number and ISIN of Unilever N.V. (Bloomberg Code: UNA NA) UBS AG will adjust the terms of the following products as of 1st July 2019. Detailed information can be found in the respective product documentation (Termsheets). ISIN Name CH0391674139 6.25% p.a. EUR Kick-In GOAL linked to worst of Henkel / Unilever / Beiersdorf / BASF CH0400505621 6.50% p.a. CHF Kick-In GOAL linked to worst of Unilever / Nestlé / Anheuser-Busch / Danone CH0420778752 5.00% p.a. EUR Kick-In GOAL linked to worst of Carrefour / Danone / Nestlé / Unilever CH0410512831 5.50% p.a. EUR Kick-In GOAL linked to worst of Nestlé / Unilever / Danone / Kellogg CH0426877814 5.50% p.a. CHF Kick-In GOAL linked to worst of Barry Callebaut / Mondelez / Danone / Unilever CH0434742570 5.50% p.a. EUR Kick-In GOAL linked to worst of Beiersdorf / Henkel / LOréal / Unilever CH0456067161 8.00% p.a. EUR Callable Kick-In GOAL linked to worst of adidas / LOréal / Unilever / Carrefour CH0468335655 6.00% p.a. EUR Callable Kick-In GOAL linked to worst of Mondelez / Danone / Unilever CH0472609830 8.00% p.a. EUR Kick-In GOAL linked to worst of Carrefour / Danone / Unilever CH0477599200 8.50% p.a. -

Q2 2010 Half-Year Financial Report Worldreginfo - C49e8129-Cd51-43E5-Af66-E15ec3c851f8 2
Q2 2010 Half-Year Financial Report WorldReginfo - c49e8129-cd51-43e5-af66-e15ec3c851f8 2 CONTENTS 3 Half-Year Management Report of the Merck Group as of June 30, 2010 3 At a glance | Highlights 4 Merck Group 8 Merck shares 9 Business sectors 11 Divisions 11 Merck Serono 13 Consumer Health Care 15 Liquid Crystals 16 Performance & Life Science Chemicals 17 Merck Millipore 18 Corporate and Other 18 Risk Report 19 Report on Expected Developments 20 Half-Year Consolidated Financial Statements as of June 30, 2010 31 Responsibility Statement 32 Executive Board | Supervisory Board | Capital structure 33 Financial calendar for 2010 | Publication contributors Cover photo: Automated distribution of miniscule particles onto microtiter plates. These are used to synthesize compound libraries for ultra-high-throughput screening in drug discovery research. WorldReginfo - c49e8129-cd51-43e5-af66-e15ec3c851f8 Half-Year Management Report of the Merck Group as of June 30, 2010 3 At a glance | Highlights AT A glaNCE Key figures – 2nd quarter 2010 Pharma Corporate Change EUR million ceuticals Chemicals and Other Total in % Total revenues 1,564.4 643.6 – 2,208.0 15.6 Gross margin 1,297.2 393.5 – 1,690.8 21.5 Research and development –302.2 –36.5 –0.1 –338.8 –0.5 Operating result 158.2 166.0 2.0 326.2 76.8 Exceptional items – – –1.2 –1.2 – Earnings before interest and tax (EBIT) 158.2 166.0 0.8 325.0 76.2 EBIT before depreciation and amortization (EBITDA) 350.0 208.6 1.3 559.9 38.4 Return on sales in % (ROS: operating result/total revenues) 10.1 25.8 – 14.8 -

Unity: Concern Over Patent Licensing Draft .•.....• 1 Proposal on Cancelling Door-To-Door Sales Deals •....•
• Issue No. 421 Report No. 311, February 11, 1977 IN THIS ISSUE page Connnunity: Concern over Patent Licensing Draft .•.....• 1 Proposal on Cancelling Door-to-Door Sales Deals •....•. 2 Briefs: Court of Auditors; Fishing Licenses .•...•....• 3 Italy: 'Social Pact' Skirts Wage Cost Issue •........•. 3 Ireland: Budget Raises Speculation on Elections ....... 4 Britain: Modest Upturn Inspires Hopes, Caution ........ S France: Employers Want Shift in Social Cost Burden ..•. 6 Germany: Labor Costs Now Higher Than in USA .•.•••••••• 7 Euro Company Scene •••......•.....•.••..•.•..•....•. , •. 7 &unity: The European Connnission is going to great lengths to alle Business Fears viate the concern of the European business connnunity, es over Patent pecially in Germany and the U.K., over a preliminary draft Licensing Draft regulation that would exempt en bloc from the Article 85(1) ban any licensing agreements containing certain restrictive clauses. The Connnission and national experts were sched uled to discuss the document again this month, before in viting connnents from national business associations. In the meantime, however, Brussels has been taken by surprise by the publication of the confidential preliminary draft in a respected German financial newspaper. Connnission offi cials are now trying to counter criticism of business rep resentatives, who have described the draft as being "too narrowly conceived, contradictory, and impractical." The regulation would relieve the parties both from no tifying the Connnission and from applying for an exemption for a restrictive licensing agreement (as they are required to do now) if the latter meets the criteria set out in the draft regulation. A block exemption would greatly ease the Connnission's work load because the applications for exemp tions have been piling up by the tens of thousands. -
Notice of the Annual Stockholders' Meeting
OF THE OF on April 27, 2012 NOTICE MEETING OF BAYER AG MEETING OF BAYER ANNUAL STOCKHOLDERS’ STOCKHOLDERS’ ANNUAL Please fill out in block letters Please Surname affix stamp First Name Street and house number Postal code Bayer AG c /o Finger Marketing Services Country Postfach 100538 41405 Neuss E-mail Germany Phone 2 CONTENTS NOTICE OF THE ANNUAL STOCKHOLDERS’ MEETING 2012 NOTICE OF THE ANNUAL STOCKHOLDERS’ MEETING 2012 KEY DATA 23 Contents Key data 2010 2011 Change AGENDA € million € million % Sales 35,088 36,528 + 4.1 1. Presentation of the adopted annual fi nancial 3 EBIT 1 2,730 4,149 + 52.0 statements and the approved consolidated fi nancial EBIT before special items 2 4,452 5,025 + 12.9 statements, the combined management report, the re- EBITDA 3 6,286 6,918 + 10.1 port of the Supervisory Board, the explanatory report by EBITDA before special items 2 7,101 7,613 + 7.2 the Board of Management on takeover-related disclosu- EBITDA margin before special items 4 20.2% 20.8% res, and the proposal by the Board of Management on the Income before income taxes 1,721 3,363 + 95.4 one-time delivery delivery regular one-time delivery delivery regular appropriation of distributable profi t for the fi scal year as an app and on the internet Net income 1,301 2,470 + 89.9 2011, as well as the resolution on the appropriation of Earnings per share (€) 5 1.57 2.99 + 90.4 distributable profi t. Core earnings per share (€) 6 4.19 4.83 + 15.3 2. -

The Resiliency Compass: Navigating Global Value Chain Disruption in an Age of Uncertainty
In Collaboration with Kearney The Resiliency Compass: Navigating Global Value Chain Disruption in an Age of Uncertainty WHITE PAPER JULY 2021 Images: Getty Images, Unsplash Contents Foreword 3 Executive summary 4 1 Disruption drives a rethink 5 2 Resilience in action 8 2.1 Getting to grips with disruption 8 2.2 Introducing the resiliency compass 8 2.3 Who are the resilience leaders? 10 3 Setting the right course with the resiliency compass 11 4 Call for action: global coordination for the long term 14 Methodology 15 Contributors 16 Acknowledgements 17 Endnotes 19 © 2021 World Economic Forum. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, including photocopying and recording, or by any information storage and retrieval system. The Resiliency Compass: Navigating Global Value Chain Disruption in an Age of Uncertainty 2 July 2021 The Resiliency Compass: Navigating Global Value Chain Disruption in an Age of Uncertainty Foreword Unlocking the future of cooperation, resilience and prosperity for global value chains. Francisco Betti Per Kristian Hong Head of Shaping the Future of Managing Director and Advanced Manufacturing and Partner, Strategic Operations Production, Member of the and World Economic Forum Executive Committee, World Relationship Lead, Kearney Economic Forum COVID-19 has kept manufacturing companies systems if both the global economy and companies beyond busy for many months and the challenges are to successfully navigate future disruptions that are far from over, from ensuring safety and may affect global value chains. security on the shop floor and facing supply and demand disruptions to accelerating digital In 2020, the World Economic Forum, in transformation and reskilling to build resilience. -

Henkel AG & Co. Kgaa
Second Supplement dated 14 January 2020 to the Prospectus dated 4 July 2019 as supplemented by the First Supplement dated 18 September 2019 This document constitutes a supplement (the "Second Supplement") within the meaning of Article 46(3) of Regulation (EU) 2017/1129 of the European Parliament and the Council of 14 June 2017 (the "Prospectus Regulation") in connection with Article 13 Luxembourg law relating to prospectuses for securities dated 10 July 2005, as amended, (Loi relative aux prospectus pour valeurs mobilières, the "Luxembourg Law 2005"), which implemented Directive 2003/71/EC of the European Parliament and the Council of 4 November 2003, as amended (the "Prospectus Directive") to the base prospectus of Henkel AG & Co. KGaA in respect of non- equity securities within the meaning of Art. 22 No. 6(4) of the Commission Regulation (EC) No. 809/2004 of 29 April 2004, as amended, ("Non-Equity Securities") (the "Debt Issuance Programme Prospectus" or the "Prospectus"). This Second Supplement is supplemental to, and must be read in conjunction with the Debt Issuance Programme Prospectus dated 4 July 2019 as supplemented by the first supplement dated 18 September 2019 (the "First Supplement") (the Prospectus together with the First Supplement, the "Supplemented Prospectus"). Henkel AG & Co. KGaA (Düsseldorf, Federal Republic of Germany) as Issuer EUR 6,000,000,000 Debt Issuance Programme (the "Programme") The Issuer has requested the Luxembourg Commission de Surveillance du Secteur Financier (the "Commission") in its capacity as competent -

Disclaimerthis Is a Publication of a Third Party
DisclaimerThis is a publication of a third party. Merck KGaA, Darmstadt, Germany is not responsible for its content. Whenever this document refers to Merck it refers to Merck KGaA, Darmstadt, Germany or the group of companies affiliated with Merck KGaA, Darmstadt, Germany. In the United States and Canada the group of companies affiliated with Merck KGaA, Darmstadt, Germany operates under individual business names (EMD Serono, MilliporeSigma, EMD Performance Materials). 2 November 2018 Corporates Merck KGaA Merck KGaAGermany, Chemicals/Pharmaceuticals STABLE Germany, Chemicals/Pharmaceuticals A- Corporate profile Ratings & Outlook Merck KGaA is a diversified chemicals/pharmaceuticals group that was founded in 1668 Corporate Ratings A-/Stable with the opening of a Merck pharmacy in Darmstadt, Germany, where the group is still Short Term Rating S-1/Stable based. The Merck family holds 70% of the voting rights with the remainder in public ownership. After several acquisitions and divestments, the group now consists of three divisions: healthcare (pharmaceuticals and consumer healthcare), life science and Analysts performance materials (centred on its global market leadership in liquid crystals). In 2015, Olaf Tölke Merck acquired the US-based life science company Sigma Aldrich for a price of +49 69 6677389 11 USD 17bn and became one of the consolidators in that industry. In pharmaceuticals, it is [email protected] a specialised, mid-sized producer of drugs, relying on two blockbuster products, Erbitux and Rebif, but has developed a new focus on immuno-oncological products, mainly Related methodology represented by its antibody avelumab. At the end of 2014, US-based big pharma Corporate Rating Methodology, company Pfizer acquired partial ownership of the molecule as well as US distribution January 2018 rights for USD 850m. -

Henkel Case A
A GRAND ENTRANCE? Li Ning’s Emergence as a Global, Chinese Brand CASE DEVELOPMENT CENTRE Henkel Customs Broker Rationalisation This case was prepared by Professor Albert Veenstra and Rebecca Chung, with the assistance of Lukas Lohrer. We would like to thank Tesse Douma at Henkel for his information and opinions. This case is based on field research data. It is written to provide material for class discussion rather than to illustrate either effective or ineffective handling of a management situation. Copyright © 2018 RSM Case Development Centre, Erasmus University. No part of this publication may be copied, stored, transmitted, reproduced or distributed in any form or medium whatsoever without the permission of the copyright owner. Please address all correspondence to [email protected]. Henkel: Customs Broker Rationalisation This case explains the customs broker rationalisation programme for European operations of the German multinational conglomerate Henkel AG & Co. KGaA since 2015. Henkel is a manufacturer of adhesive, beauty care, laundry and home care products with 170 production sites in 57 countries and sales in over 150 countries. The case shows how the company’s Global Customs Management and Export Compliance project team in Amsterdam designed and launched the programme, and invited customs broker candidate firms for a final selection in summer 2016. What should the evaluation criteria be for selecting the right broker? Introduction In Summer 2016, an important recommendation was about to be made by Head of the Global Customs Management and Export Control project unit (hereafter “the Compliance team”) of the multinational conglomerate Henkel AG & Co. KGaA (Henkel). He wanted his management superiors to significantly change the company’s approach towards global trade management. -

Dr. Holger Hofmeister
Dr. Holger Hofmeister Partner, Frankfurt Mergers and Acquisitions Dr. Holger Hofmeister advises corporate clients and private equity firms in connection with acquisitions, divestitures, public takeovers, joint ventures, and other M&A and corporate matters, including capital markets-related issues. In addition, he advises on corporate governance-related matters and corporate restructurings. Dr. Hofmeister was named in WirtschaftsWoche as one of the best corporate lawyers in Germany. In addition, he has been named by both Handelsblatt and WirtschaftsWoche as one of Germany’s top M&A advisors. He also is listed as recommended lawyer in JUVE and was listed in Best Lawyers in Germany 2021. Dr. Hofmeister’s representative matters include advising, among others: - Adevinta ASA in its US$9.2 billion acquisition of eBay Classifieds Group from eBay Inc.; T: 49.69.74220.117 - Airbnb, Inc. in its acquisition of Germany-based fewoVista S.L. & Co. KG and Vacaleo GmbH; F: 49.69.74220.417 - Apax Partners in connection with: [email protected] • its proposed US$8.5 billion bid for ISS/AS, a facilities management provider, from Goldman Sachs Capital Partners and EQT Partners AB; and Education • the US$420 million sale of Versatel AG, a provider of telecommunications and informa- Second State Exam, Appellate Court tion technology services, to Kohlberg Kravis Roberts & Co., a private equity firm; of Duesseldorf, 2001 - Black Diamond Capital Management, L.L.C., along with InvestIndustrial, in their Dr. jur., University of Regensburg, 1999 US$425 million acquisition of the phenolic specialty resin, hexamine and European forest products resins businesses of Hexion Inc.; First State Exam, University of Regensburg, 1998 - Braskem S.A. -
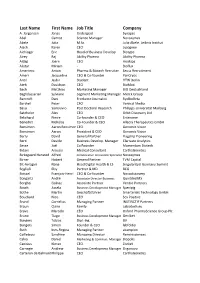
Last Name First Name Job Title Company A
Last Name First Name Job Title Company A. Jürgensen Jonas Undergrad Synapse Abel Gernot Science Manager Novozymes Abele Julia M.Sc. Julia Abele, Leibniz Institut Aiach Karen CEO Lysogene Aichinger Eric Head of Business Develop. Biotype Airey Raj Ability Pharma Ability Pharma Aldag Joern CEO Hookipa Alistar Miriam - Bioflux Ameniero Anton Pharma & Biotech Recruiter Seuss Recruitment Ameri JacQueline CEO & Co-founder PanCryos Antil Jasbir Student HTW Berlin Ateh Davidson CEO BioMoti Bach Matthias Marketing Manager BIO Deutschland Baghdassarian Sylviane Segment Marketing Manager Merck Group Bancroft Dani Freelance Journalist SynBioBeta Barthel Peter CFO Vertical Media Basu Somsuvro Post Doctoral Research Philipps-Universität Marburg Batchelor Alex CEO Orbit Discovery Ltd Belichard Pierre Co-founder & CEO Enterome Benedict Nicholas Co-Founder & CEO Allecra Therapeutics GmbH Bensimon Aaron BensimonCEO Genomic Vision Bensimon Aaron President & CEO Genomic Vision Berry David General Partner Flagship Pioneering Berti Davide Business Develop. Manager Clarivate Analytics Besse Joël Co Founder Momentum Biotech Bidani Aroussi Medical Consultant CarthaGenetics Birkegaard Hansted Alfred Collaborative Innovation Specialist Novozymes Birner Hubert General Partner TVM Capital Bit-Avragim Nana Head Digital Health & LS SingularityU Germany Summit Boglioli Elsy Partner & MD BCG Boissel François-Henri CEO & Co-founder Novadiscovery Bongartz André Associate Director Business QuintilesIMS Borghei Golnaz Associate Partner Ventac Partners Bosch Assela Business Development Manager Specipig Bothe Martin Geschäftsführer Smarterials Technology GmbH Bouchard Nico CEO Sex Positive Brand Cornelius Managing Partner INSTINCTIF Partners Braun Claire Family Labiotech.eu Bravo Marcelo CEO Oxford PharmaScience Group Plc Bruno Tiago Business Development Manager GenIbet Bungers Tobias Dipl.-Ing. BV Bungers Simon Founder & CEO labfolder Buth Ines-Regina Managing Partner akampion Cailleteau Sandrine Managing Director Reminisciences Calvo Daniel Marketing & Commun. -

2.2.2. List of Corporate Offices and Directorships of the Directors Exercised at 31 December 2020
CORPORATE GOVERNANCE Composition of the Board of Directors 2.2.2. List of corporate offices and directorships of the Directors exercised at 31 December 2020 JEAN-PAUL AGON Chairman and Chief Executive Officer French Age: 64 Joined the L’Oréal Group in 1978. Following an international career as Chief Executive Officer of the Consumer Products 2 Division in Greece and of L’Oréal Paris in France, International Managing Director of Biotherm, Chief Executive Officer of L’Oréal Germany, Chief Executive Officer of the Asia Zone, Chairman and Chief Executive Officer of L’Oréal USA, Jean-Paul Agon was appointed as Deputy Chief Executive Officer of L’Oréal in 2005, and then Chief Executive Officer in April 2006 and finally Chairman and Chief Executive Officer in 2011. A Director of L’Oréal since 2006, he is also the Chairman of the Strategy and Sustainability Committee. Jean-Paul Agon is also the Chairman of the L’Oréal Corporate Foundation and Director of Air Liquide. By decision of the Board of Directors following the 2021 Annual General Meeting, Jean-Paul Agon will hold the office of Chairman of the Board of Directors without assuming the office of Chief Executive Officer as from 1 May 2021. EXPIRY DATE OF TERM OF OFFICE: 2022 ● Professional address: L’Oréal – 41, rue Martre – 92117 Clichy Cedex – France ● Holds 1,365,432 L’Oréal shares OTHER CORPORATE OFFICES AND DIRECTORSHIPS HELD French companies Air Liquide S.A.* Director Other L’Oréal Corporate Foundation Chairman of the Board L’Oréal Fund for Women Chairman of the Board Raisesherpas Director French Association of Private Enterprises (AFEP) Director French Institute of International Relations (IFRI) Director CORPORATE OFFICES AND DIRECTORSHIPS HELD OVER THE LAST FIVE YEARS AND EXPIRED None * Listed company. -
Invitation to the Ordinary Annual General Meeting on April 1, 2021 (Virtual Annual General Meeting)
This English translation is provided for convenience only. The German text shall be the sole legally binding version. Invitation to the Ordinary Annual General Meeting on April 1, 2021 (Virtual Annual General Meeting) Beiersdorf Aktiengesellschaft, Hamburg Wertpapier-Kennnummer 520000 ISIN DE0005200000 HEALING OINTMENT 2 Beiersdorf AG Invitation Beiersdorf Aktiengesellschaft share- holders are hereby invited to attend the Company’s Annual General Meeting to be held on Thursday, April 1, 2021, at 10.30 a.m. (CEST) Against the background of the still ongoing coronavirus pandemic and the importance of protecting all parties involved, this year’s Annual General Meeting will again be held as a virtual meeting without the physical presence of the shareholders or their proxy- holders Overview containing information in accordance with Section 125 of the German Stock Corporation Act (AktG) in conjunction with Table 3 of the Implementing Regulation (EU) 2018/12122 A. Specification of the message 1. Unique identifier of the event: Beiersdorf_AGM_2021 2. Type of message: Notice of Annual General Meeting B. Specification of the issuer 1. ISIN: DE0005200000 2. Name of issuer: Beiersdorf Aktiengesellschaft C. Specification of the meeting 1. Date of the General Meeting: 2021-04-01 2. Time of the General Meeting: 08:30 a.m. UTC [corresponds to 10:30 a.m. CEST] 3. Type of General Meeting: Annual General Meeting 4. Location of the General Meeting: https://www .beiersdorf.com/annual-general-meeting 5. Record Date: 2021-03-10 [Record Date within the meaning of § 123 Abs. 4 AktG and § 18 Abs. 2 of the Articles of Association of Beiersdorf Aktiengesellschaft is March 11, 2021] 6.