FOR JEE MAIN & ADVANCED Class 11 2017-18
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
Nomenclature of Organic Compounds Consists of the Following Two Systems
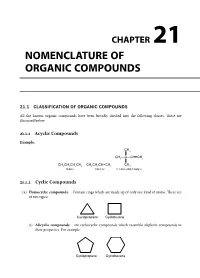
CHAPTER NOMENCLATURE OF 21 ORGANIC COMPOUNDS 21.1 CLASSIFICATION OF ORGANIC COMPOUNDS All the known organic compounds have been broadly divided into the following classes. These are discussed below 21.1.1 Acyclic Compounds Example: &+ &+²&²&ŁŁŁ&+ &+&+&+&+ &+&+&+ &+ &+ %XWDQH %XWHQH 'LPHWK\OEXW\QH 21.1.2 Cyclic Compounds (a) Homocyclic compounds: Contain rings which are made up of only one kind of atoms. These are of two types: &\FORSURSDQH &\FOREXWDQH (i) Alicyclic compounds: are carbocyclic compounds which resemble aliphatic compounds in their properties. For example &\FORSURSDQH &\FORKH[DQH Chemistry at a Glance Final.pdf 251 4/1/2014 12:26:17 PM 21.246 Chemistry at a Glance (ii) Aromatic compounds: These are also called benzenoid compounds or arenes. For example 1DSKWKDOHQH $QWKUDFHQH 3KHQDQWKUHQH %LSKHQ\ORU'LSKHQ\O (b) Heterocyclic compounds: Cyclic compounds containing one or more heteroatoms (e.g., O,N,S, etc.) in the ring are called heterocyclic compounds. These are of two types: (i) Alicyclic heterocyclic compounds: Heterocyclic compounds which resemble aliphatic com- pounds in their properties are called alicyclic heterocyclic compounds, For example 2 2 2 1 1 2 + + 2[LUDQHRU 7HWUDK\GURIXUDQ 'LR[DQH 3\UUROLGLQH 'LR[DQH (SR[\HWKDQH 7+) (ii) Aromatic heterocyclic compounds. Heterocyclic compounds which resemble benzene and other aromatic compounds in most of their properties 2 1 6 + 1 are called aromatic heterocyclic compounds. )XUDQ )XUDQ 7KLRSKHQH 3\ULGLQH For example 21.2 SYSTEM OF NOMENCLATURE FOR ORGANIC COMPOUNDS Nomenclature of organic compounds consists of the following two systems. 21.2.1 Trivial System or Derived System In the trivial system, the name of the compound could indicate a compound source from which it is derived. -

Recording Sheets Containing Amino Acids, Hydroxy Acids, and Polycarboxyl Compounds
Office europeen des brevets (fi) Publication number : 0 667 246 A1 @ EUROPEAN PATENT APPLICATION @ Application number: 95300919.8 @ Int. CI.6: B41M 5/00, D21H 17/14 (22) Date of filing : 14.02.95 (30) Priority : 15.02.94 US 196679 @ Inventor : Malhotra, Shadi L. 4191 Taffey Crescent Mississauga, Ontario LSL2A6 (CA) (43) Date of publication of application : 16.08.95 Bulletin 95/33 (74) Representative : Reynolds, Julian David et al Rank Xerox Ltd @ Designated Contracting States : Patent Department DE FR GB Parkway Marlow Buckinghamshire SL7 1YL (GB) @ Applicant : XEROX CORPORATION Xerox Square Rochester New York 14644 (US) (54) Recording sheets containing amino acids, hydroxy acids, and polycarboxyl compounds. (57) A recording sheet which comprises a paper substrate and a material selected from the group consisting of monomeric amino acids, monomeric hydroxy acids, monomeric polycarboxyl com- pounds, and mixtures thereof. Another embodiment of the present invention is directed to a recording sheet which comprises a substrate and a material selected from the group consisting of monomeric amino acids, monomeric hydroxy acids, and mixtures thereof. CO CM h- (O CO LU Jouve, 18, rue Saint-Denis, 75001 PARIS EP 0 667 246 A1 The present invention is directed to recording sheets, such as transparency materials, filled plastics, pa- pers, and the like. More specifically, the present invention is directed to recording sheets particularly suitable for use in ink jet printing processes. South African Patent Application 924,610 discloses a transparent -

Chemicals Subject to TSCA Section 12(B) Export Notification Requirements (January 16, 2020)
Chemicals Subject to TSCA Section 12(b) Export Notification Requirements (January 16, 2020) All of the chemical substances appearing on this list are subject to the Toxic Substances Control Act (TSCA) section 12(b) export notification requirements delineated at 40 CFR part 707, subpart D. The chemicals in the following tables are listed under three (3) sections: Substances to be reported by Notification Name; Substances to be reported by Mixture and Notification Name; and Category Tables. TSCA Regulatory Actions Triggering Section 12(b) Export Notification TSCA section 12(b) requires any person who exports or intends to export a chemical substance or mixture to notify the Environmental Protection Agency (EPA) of such exportation if any of the following actions have been taken under TSCA with respect to that chemical substance or mixture: (1) data are required under section 4 or 5(b), (2) an order has been issued under section 5, (3) a rule has been proposed or promulgated under section 5 or 6, or (4) an action is pending, or relief has been granted under section 5 or 7. Other Section 12(b) Export Notification Considerations The following additional provisions are included in the Agency's regulations implementing section 12(b) of TSCA (i.e. 40 CFR part 707, subpart D): (a) No notice of export will be required for articles, except PCB articles, unless the Agency so requires in the context of individual section 5, 6, or 7 actions. (b) Any person who exports or intends to export polychlorinated biphenyls (PCBs) or PCB articles, for any purpose other than disposal, shall notify EPA of such intent or exportation under section 12(b). -

Federal Register/Vol. 84, No. 55/Thursday, March 21, 2019/Notices
Federal Register / Vol. 84, No. 55 / Thursday, March 21, 2019 / Notices 10499 An agency may not conduct or sponsor impose radionuclide dose and/or ENVIRONMENTAL PROTECTION and a person is not required to respond emission limits, respectively, to AGENCY to a collection of information unless it underground uranium mines, elemental [EPA–HQ–OPPT–2018–0408; FRL 9990–07] displays a currently valid OMB control phosphorous plants, phosphogypsum number. stacks, and uranium mill tailings Certain New Chemical Substances; DATES: Additional comments may be impoundments. Facilities must measure Receipt and Status Information for submitted on or before April 22, 2019. their radionuclide emissions, perform September 2018 ADDRESSES: Submit your comments, analysis or calculations per EPA referencing Docket ID Number EPA– procedure, and report the results to the AGENCY: Environmental Protection HQ–OAR–2003–0085–0014, to (1) EPA EPA. Agency (EPA). online using www.regulations.gov (our Information collected is used by the ACTION: Notice. preferred method), by email to a-and-r- EPA to ensure that public health SUMMARY: EPA is required under the [email protected] or by mail to: EPA continues to be protected from the Toxic Substances Control Act (TSCA), Docket Center, Environmental hazards of airborne radionuclides by as amended by the Frank R. Lautenberg Protection Agency, Mail Code 28221T, compliance with these standards. Chemical Safety for the 21st Century 1200 Pennsylvania Ave. NW, Compliance is demonstrated through Act, to make information publicly Washington, DC 20460, and (2) OMB via emissions testing and dose calculation _ available and to publish information in email to oira [email protected]. -

C:\Inetpub\Wwwroot\Iqp\CHE 310\Nomall.Wpd
9 Additional Guidelines for Naming Cycloalkanes and Cycloalkenes 1. Cycloalkanes and cycloalkenes are named as for alkanes and alkenes except that they include the prefix ‘cyclo’ in the name. 2. The ring is numbered either clockwise or anticlockwise so that the substituents are assigned the lowest number possible. 3. For mono-substituted cycloalkanes, the site of the substituent is assumed to be C-1 and it is not included in the name. 4. For cycloalkenes, the site of the alkene is assumed to be C-1. 5. When the ring is a substituent on a long chain, it is named a cycloalkyl substituent. If there are other substituents on such a ring, it is treated in the same way as the branched substituents that were discussed earlier. Examples 1. Give the IUPAC name for: 3 2 1 4 5 6 STRATEGY/OBSERVATIONS When working with cyclic molecules, I suggest that you redraw the structure using the line angle representation,© COPYRIGHTED but you should MATERIAL number the carbons J.M.E. atoms QUIRKE on the ring 9/1/1999, as is drawn above. Fill out the nomenclature template OBSERVATION IMPLICATION Parent Group and Site cyclic alkane cyclo....ane Longest Carbon Chain/Ring 6-membered ring cyclohexane # C=C or C/C bonds and Site None Final Word cyclohexane Substituents and Sites 2 CH3's at C-1 & C-3 1,3-dimethyl Alphabetizing substituents 1,3-dimethyl SOLUTION Compound is 1,3-Dimethylcyclohexane 10 2. Give the IUPAC name for: 3 2 4 5 1 6 7 STRATEGY/OBSERVATIONS When numbering a cycloalkene, select as C-1 the carbon of the double bond that will generate the lowest numbers for sites of the ring substituents. -

Tommunom U Dit Arti Mitte
TOMMUNOMUS009822353B2 U DITARTI MITTE (12 ) United States Patent (10 ) Patent No. : US 9 ,822 , 353 B2 Buechler et al . ( 45 ) Date of Patent: Nov . 21 , 2017 ( 54 ) PEGYLATED ASPARTYL - TRNA 5 ,753 , 480 A 5 / 1998 Lawlor SYNTHETASE POLYPEPTIDES 5 , 756 , 327 A 5 / 1998 Sassanfar et al . 5 ,759 ,833 A 6 / 1998 Shiba et al. 5 , 776 ,749 A 7 / 1998 Hodgson et al. (71 ) Applicant : a Tyr Pharma, Inc ., San Diego , CA 5 , 795 ,757 A 8 / 1998 Hodgson et al . (US ) 5 , 798 , 240 A 8 / 1998 Martinis et al . 5 , 801, 013 A 9 / 1998 Tao et al . (72 ) Inventors : Ying Ji Buechler, Carlsbad , CA (US ); 5 , 866 , 390 A 2 / 1999 Lawlor 5 , 885 , 815 A 3 / 1999 Sassanfar et al . Chi - Fang Wu , San Diego , CA (US ) ; 5 , 928 , 920 A 7 / 1999 Hodgson et al. Jeffrey Greve , Berkeley , CA (US ); 5 , 939 , 298 A 8 / 1999 Brown et al. John D . Mendlein , Encinitas , CA (US ) 6 , 225, 060 B1 5 / 2001 Clark et al. 6 , 255, 090 B1 7 / 2001 Famodu et al. ( 73 ) Assignee : aTyr Pharma, Inc ., San Diego , CA 6 , 265 ,188 B1 7 / 2001 Brown et al. (US ) 6 , 428 , 960 B1 8 / 2002 Clark et al. 6 , 548 , 060 B1 4 / 2003 Kim 6 , 696, 619 B1 2 / 2004 Famodu et al. ( * ) Notice: Subject to any disclaimer, the term of this 6 ,852 , 512 B2 2 / 2005 Choi et al. patent is extended or adjusted under 35 6 , 903 , 189 B2 6 /2005 Schimmel et al. U . S . -

Chemicals Subject to TSCA Section 12(B) Export Notification Requirements (January 16, 2020)
Chemicals Subject to TSCA Section 12(b) Export Notification Requirements (January 16, 2020) All of the chemical substances appearing on this list are subject to the Toxic Substances Control Act (TSCA) section 12(b) export notification requirements delineated at 40 CFR part 707, subpart D. The chemicals in the following tables are listed under three (3) sections: Substances to be reported by Notification Name; Substances to be reported by Mixture and Notification Name; and Category Tables. TSCA Regulatory Actions Triggering Section 12(b) Export Notification TSCA section 12(b) requires any person who exports or intends to export a chemical substance or mixture to notify the Environmental Protection Agency (EPA) of such exportation if any of the following actions have been taken under TSCA with respect to that chemical substance or mixture: (1) data are required under section 4 or 5(b), (2) an order has been issued under section 5, (3) a rule has been proposed or promulgated under section 5 or 6, or (4) an action is pending, or relief has been granted under section 5 or 7. Other Section 12(b) Export Notification Considerations The following additional provisions are included in the Agency's regulations implementing section 12(b) of TSCA (i.e. 40 CFR part 707, subpart D): (a) No notice of export will be required for articles, except PCB articles, unless the Agency so requires in the context of individual section 5, 6, or 7 actions. (b) Any person who exports or intends to export polychlorinated biphenyls (PCBs) or PCB articles, for any purpose other than disposal, shall notify EPA of such intent or exportation under section 12(b). -

Basic Principles of Organic Chemistry
8. BASIC PRINCIPLES OF ORGANIC CHEMISTRY 1. INTRODUCTION What is organic chemistry? Organic chemistry is the study of most carbon compounds with the exception of a few (e.g., CO2 and carbonate salts). While inorganic chemistry deals with the study of all other compounds. 2. PROPERTIES OF ORGANIC COMPOUNDS In general, organic compounds. (a) Are far more in number than inorganic compounds. This is due to the catenation property of the C atom. Carbon has the ability to form bonds with almost every other element (Other than the noble gases), forming long chains as well as ring compounds. Moreover, C compounds exist as many isomers. (b) React more slowly and require higher temperatures for reactions to take place. (c) Are less stable and sometimes decompose on heating to compounds of lower energy levels. (d) Undergo more complex reactions and produce more side reaction products. (e) Are largely insoluble in water. (f) Have generally lower melting and boiling points. (g) Are classified into families of compounds such as carboxylic acids, which have similar reactive groups and chemical properties. (h) Are mostly obtained from animals or plants as opposed to the mineral origin of inorganic compounds. 3. CLASSIFICATION OF ORGANIC COMPOUNDS They are classified follows: Classification of organic compounds is basically based on the functional group. The chemical properties of compound depends on the properties of the functional group present in it. The rest of the molecule simply affects the physical properties, e.g., m.p., b.p., density etc. and has very little effect on its chemical properties. 8.2 | Basic Principles of Organic Chemistry Organic compounds Open chain or acrylic or Closed chain or aliphatic compounds, alicyclic or cyclic or ring compounds e.g.