Chemicals Subject to TSCA Section 12(B) Export Notification Requirements (January 16, 2020)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

CO2 Capture from Flue Gas Using Amino Acid Salt Solutions
CO2 capture from flue gas using amino acid salt solutions Jacco van Holst, Patricia. P. Politiek, John P. M. Niederer, Geert F. Versteeg* University of Twente, Faculty Science and Technology (UT TNW), P.O. Box 217, 7500 AE Enschede, The Netherlands Abstract An initial kinetic study was performed on the reaction of CO2 with various potassium amino acid salt solutions at 298 K. Kinetics were measured at 0.5 kmol/m3, reason for which only apparent ki- netic constants are presented. The results were compared with the work of Kumar et al. [1] and Penny and Ritter [2]. Keywords: CO2, flue gas treatment, kinetics, potassium amino acid salt solutions Introduction One of the most alarming global environmental problems of today is the increase of the natural greenhouse effect. This problem is mainly caused by the increasing atmospheric carbon dioxide concentration due to the burning of fossil fuels for power generation. To reduce these problems, the carbon dioxide emissions from flue and fuel gases produced in combustion and gasification proc- esses in power plants have to be decreased by efficiency improvements and carbon dioxide capture. The removal of acid gases such as carbon dioxide, H2S or COS by absorption in aqueous alkanola- mine solutions is widely used in the chemical industry. Carbon dioxide reacts with primary and sec- ondary amines, reaching an equilibrium of carbamate, bicarbonate, and carbonate species. The ini- tial absorption reaction is the formation of the carbamate, which can then undergo hydrolysis to the bicarbonate and, if conditions such as pH are suitable, the carbonate species. The degree of hydroly- sis of the carbamate is determined by parameters such as amine concentration, solution pH, and the chemical stability of the carbamate [3]. -

SALTS of FATTY ACIDS
SALTS of FATTY ACIDS Prepared at the 33rd JECFA (1988), published in FNP 38 (1988) and in FNP 52 (1992). Metals and arsenic specifications revised at the 55th JECFA (2000). An ADI 'not specified' was established at the 33rd JECFA (1988) SYNONYMS INS No. 470 DEFINITION These products consist of calcium, potassium or sodium salts of commercial myristic, oleic, palmitic, stearic, acids or mixtures of these acids from edible fats and oils. The article of commerce can be further specified by: - saponification value, - solidification point for the fatty acids obtained from the salts, - iodine value, - residue on ignition including assay of the cation, and - moisture content Assay Not less than 95% total fatty acid salts, dry weight basis DESCRIPTION Hard, white or faintly yellowish, somewhat glossy and crystalline solids or semi-solids or white or yellowish-white powder FUNCTIONAL USES Anticaking agent, emulsifier CHARACTERISTICS IDENTIFICATION Solubility (Vol. 4) Potassium and sodium salts are soluble in water and ethanol; calcium salts are insoluble in water, ethanol and ether Test for cations Heat 1 g of the sample with a mixture of 25 ml of water and 5 ml of hydrochloric acid. Fatty acids are liberated, floating as a solid or oil layer on the surface which is soluble in hexane. After cooling, aqueous layer is decanted and evaporated to dryness. Dissolve the residue in water and test for the appropriate cation. Fatty acid composition Using the Method of Assay, identify the individual fatty sample. The fatty acid(s) in primary abundance should conform to those declared on the label of the product PURITY Free fatty acids Not more than 3% Measure free fatty acids as directed in the method Free Fatty Acids. -

RIFM Fragrance Ingredient Safety Assessment, 2-Isopropyl-4- Methylanisole, CAS Registry Number 31574-44-4
Food and Chemical Toxicology 110 (2017) S545eS551 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Short review RIFM fragrance ingredient safety assessment, 2-isopropyl-4- methylanisole, CAS Registry Number 31574-44-4 * A.M. Api a, , D. Belsito b, D. Botelho a, D. Browne a, M. Bruze c, G.A. Burton Jr. d, J. Buschmann e, M.L. Dagli f, M. Date a, W. Dekant g, C. Deodhar a, M. Francis a, A.D. Fryer h, K. Joshi a,S.LaCavaa, A. Lapczynski a, D.C. Liebler i,D.O’Brien a, R. Parakhia a,A.Patela, T.M. Penning j, G. Ritacco a, J. Romine a, D. Salvito a, T.W. Schultz k, I.G. Sipes l, Y. Thakkar a, E.H. Theophilus a, A.K. Tiethof a, Y. Tokura m, S. Tsang a, J. Wahler a a Research Institute for Fragrance Materials, Inc., 50 Tice Boulevard, Woodcliff Lake, NJ 07677, USA b Columbia University Medical Center, Department of Dermatology, 161 Fort Washington Ave., New York, NY 10032, USA c Malmo University Hospital, Department of Occupational & Environmental Dermatology, Sodra Forstadsgatan 101, Entrance 47, Malmo SE-20502, Sweden d School of Natural Resources & Environment, University of Michigan, Dana Building G110, 440 Church St., Ann Arbor, MI 58109, USA e Fraunhofer Institute for Toxicology and Experimental Medicine, Nikolai-Fuchs-Strasse 1, 30625 Hannover, Germany f University of Sao Paulo, School of Veterinary Medicine and Animal Science, Department of Pathology, Av. Prof. dr. Orlando Marques de Paiva, 87, Sao Paulo CEP 05508-900, Brazil g University of Wuerzburg, Department of Toxicology, Versbacher Str. -

DP70460.Pdf (4.893Mb)
HEAT CHAHGES ACCGKPABYIIJG ABSGRFTIOH EQUILIBRIA IB SOLTJTIQH. D. C. UCETSBVAUfER C h e w . LD Sill ,/V\7 od L VVC\ I If) <2 D-C. C o ' :o Thesim submitted to the Faculty of the Graduate School of the TJnivsrsity of Maryland in partial fulfillment of the requirements for the degree of Doctor of Phil osophy* A 1 9 fc 6 .. UMI Number: DP70460 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Dissertation Publishing UMI DP70460 Published by ProQuest LLC (2015). Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 ACKHQWLXDGMEHT The writer wishes to express hie appreciation and thaiifcs to Dr* Hell S. Gordon, Head of the Depart ment of Chemistry of the University of Maryland who dlreetcd this work and gate helpful suggestions and advice* TABLE OF G CH U M S X* Introduct 1 q h -—-*——— «*►»»««*«*»*«»»*— «■»»*-«««»»» x . Historical He view-----— ----- —■—— — — Z Heat of Absorption of Liquids art Gases- 1 4 Heat of Coagulation --- -- ™ -i^ * General Methods and Materials--— — — — — 2H of G els— 38H ----- - --- — ;S6 ------ Froeebnre——— ——-——32 If# Experiiaenial Hes’nlts-— --— — — — — ---- g*> T. Hubimavy C oixdua i OHS— —— — -— —— 61 FX* Lit eratare Gi ted :——— Introduction Adscript ion has for a long time teen a well recognised phenomenon and numerous investigations have been mad® on the nature and magnitude of the energy changes involved* Some of the measurementa on the adsorption of gases have shown ti&se changes to be enormous. -
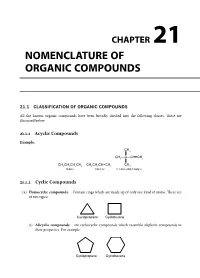
Nomenclature of Organic Compounds Consists of the Following Two Systems
CHAPTER NOMENCLATURE OF 21 ORGANIC COMPOUNDS 21.1 CLASSIFICATION OF ORGANIC COMPOUNDS All the known organic compounds have been broadly divided into the following classes. These are discussed below 21.1.1 Acyclic Compounds Example: &+ &+²&²&ŁŁŁ&+ &+&+&+&+ &+&+&+ &+ &+ %XWDQH %XWHQH 'LPHWK\OEXW\QH 21.1.2 Cyclic Compounds (a) Homocyclic compounds: Contain rings which are made up of only one kind of atoms. These are of two types: &\FORSURSDQH &\FOREXWDQH (i) Alicyclic compounds: are carbocyclic compounds which resemble aliphatic compounds in their properties. For example &\FORSURSDQH &\FORKH[DQH Chemistry at a Glance Final.pdf 251 4/1/2014 12:26:17 PM 21.246 Chemistry at a Glance (ii) Aromatic compounds: These are also called benzenoid compounds or arenes. For example 1DSKWKDOHQH $QWKUDFHQH 3KHQDQWKUHQH %LSKHQ\ORU'LSKHQ\O (b) Heterocyclic compounds: Cyclic compounds containing one or more heteroatoms (e.g., O,N,S, etc.) in the ring are called heterocyclic compounds. These are of two types: (i) Alicyclic heterocyclic compounds: Heterocyclic compounds which resemble aliphatic com- pounds in their properties are called alicyclic heterocyclic compounds, For example 2 2 2 1 1 2 + + 2[LUDQHRU 7HWUDK\GURIXUDQ 'LR[DQH 3\UUROLGLQH 'LR[DQH (SR[\HWKDQH 7+) (ii) Aromatic heterocyclic compounds. Heterocyclic compounds which resemble benzene and other aromatic compounds in most of their properties 2 1 6 + 1 are called aromatic heterocyclic compounds. )XUDQ )XUDQ 7KLRSKHQH 3\ULGLQH For example 21.2 SYSTEM OF NOMENCLATURE FOR ORGANIC COMPOUNDS Nomenclature of organic compounds consists of the following two systems. 21.2.1 Trivial System or Derived System In the trivial system, the name of the compound could indicate a compound source from which it is derived. -

Arenechromium Tricarbonyl Complexes: Conformational
η6 – ARENECHROMIUM TRICARBONYL COMPLEXES: CONFORMATIONAL ANALYSIS, STEREOCONTROL IN NUCLEOPHILIC ADDITION AND APPLICATIONS IN ORGANIC SYNTHESIS by HARINANDINI PARAMAHAMSAN Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Thesis Advisor: Prof. Anthony J. Pearson Department of Chemistry CASE WESTERN RESERVE UNIVERSITY May 2005 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the dissertation of Harinandini Paramahamsan candidate for the Ph.D. degree*. (signed) Prof. Philip P. Garner (Chair of the Committee, Department of Chemistry, CWRU) Prof. Anthony J. Pearson (Department of Chemistry, CWRU) Prof. Fred L. Urbach (Department of Chemistry, CWRU) Dr. Zwong-Wu Guo (Department of Chemistry, CWRU) Dr. Stuart J. Rowan (Department of Macromolecular Science and Engineering, CWRU) Date: 14th January 2005 *We also certify that written approval has been obtained for any propriety material contained therein. To Amma, Naina & all my Teachers Table of Contents List of Tables………………………………………………………………………..……iv List of Figures…………………………………………………………………….…........vi List of Schemes…………………………………………………………………….….….ix List of Equations………………………………………………………...……….……….xi Acknowledgements………………………………………………………….…..……….xii List of Abbreviations……………………………………………………………………xiv Abstract………………………………………………………………………………….xvi CHAPTER I........................................................................................................................ 1 I.1 Structure and Bonding ........................................................................................... -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

RIFM Fragrance Ingredient Safety Assessment, Anisyl Alcohol, CAS Registry T Number 105-13-5 A.M
Food and Chemical Toxicology 134 (2019) 110702 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Short Review RIFM fragrance ingredient safety assessment, anisyl alcohol, CAS registry T number 105-13-5 A.M. Apia, D. Belsitob, S. Bisertaa, D. Botelhoa, M. Bruzec, G.A. Burton Jr.d, J. Buschmanne, M.A. Cancellieria, M.L. Daglif, M. Datea, W. Dekantg, C. Deodhara, A.D. Fryerh, S. Gadhiaa, L. Jonesa, K. Joshia, A. Lapczynskia, M. Lavellea, D.C. Liebleri, M. Naa, D. O'Briena, A. Patela, T.M. Penningj, G. Ritaccoa, F. Rodriguez-Roperoa, J. Rominea, N. Sadekara, D. Salvitoa, ∗ T.W. Schultzk, F. Siddiqia, I.G. Sipesl, G. Sullivana, , Y. Thakkara, Y. Tokuram, S. Tsanga a Research Institute for Fragrance Materials, Inc., 50 Tice Boulevard, Woodcliff Lake, NJ, 07677, USA b Member Expert Panel, Columbia University Medical Center, Department of Dermatology, 161 Fort Washington Ave., New York, NY, 10032, USA c Member Expert Panel, Malmo University Hospital, Department of Occupational & Environmental Dermatology, Sodra Forstadsgatan 101, Entrance 47, Malmo SE, 20502, Sweden d Member Expert Panel, School of Natural Resources & Environment, University of Michigan, Dana Building G110, 440 Church St., Ann Arbor, MI, 58109, USA e Member Expert Panel, Fraunhofer Institute for Toxicology and Experimental Medicine, Nikolai-Fuchs-Strasse 1, 30625, Hannover, Germany f Member Expert Panel, University of Sao Paulo, School of Veterinary Medicine and Animal Science, Department of Pathology, Av. Prof. dr. Orlando Marques de Paiva, 87, Sao Paulo, CEP 05508-900, Brazil g Member Expert Panel, University of Wuerzburg, Department of Toxicology, Versbacher Str. -

United States Patent Office Patented July 18, 1972 1
3,677,770 United States Patent Office Patented July 18, 1972 1. 2 It will be apparent that those fusible sugars which may 3,677,770 be employed have a melting or fusion point below their CARBONATED CANDY Frank Witzel, Spring Valley, N.Y., assignor to decomposition temperature, and that no substantial de Beech-Nut, Inc., New York, N.Y. composition occurs at the melting or fusion temperature No Drawing. Filed Oct. 7, 1970, Ser. No. 78,910 which would interfere with fusion, melting, or solidifica Int, Cl, A23g 3/00 tion on cooling. U.S. C. 99-134 R 2 Claims Although as will be apparent from this disclosure, the fusible sugars which may be used in the practice of this invention include those which have a melting or fusing ABSTRACT OF THE DISCLOSURE 0 point which falls within a wide range, the preferred mate rials will be those having a melting or fusing point at The off-taste present in effervescent hard candy due to temperatures of from slightly above room temperature unreacted food acidulant as well as salt formed by the to about 300 F. (149 C.). reaction of the efferverscent factors, i.e., leavening agent The fusible sugars which may be employed in the prac and acidulant, is overcome by incorporating a small tice of this invention include sugars and their derivatives amount of a saccharin into the candy. such as sugar alcohols and sugar acids. Typical fusible monosaccharide sugars include glucose, fructose (levu lose), invert sugar (chemically equal parts of glucose BACKGROUND OF THE INVENTION and fructose), arabinose, etc. -

Metals and Acids Key Revision Facts • to Test for Hydrogen Gas- Place a Lighted Spill Near the Gas and Hear a ‘Squeaky Pop’
Metals and Acids Key Revision Facts • To test for hydrogen gas- place a lighted spill near the gas and hear a ‘squeaky pop’. • Potassium, sodium and magnesium are all examples of reactive metals. • Copper, lead and gold are all examples of unreactive metals. • When reactive metals are placed in acids they will react violently with lots of gas given off. Unreactive metals do not react with the acid. • The equation for the reaction between a metal and an acid is: metal + acid salt + hydrogen Zn + HCl sZnCl + H • State symbols Solid (s) Liquid (l) Gas (g) Aqueous - a substance dissolved in water (aq) • Magnesium and iron filings react vigorously with air • Group 1 metals react with water to produce hydroxides and hydrogen. • sodium + water sodium hydroxide + hydrogen • Some metals like magnesium react slowly with cold water but will react quickly with steam. • The reactivity series lists in order, how reactive metals are: K Potassium Most reactive Na Sodium Ca Calcium Mg Magnesium Al Aluminium Zn Zinc Fe Ferum Increasingly reactive Sn Tin Pb Lead Cu Copper Hg Mercury Ag Silver Least Au Gold reactive Metals and Acids Key Revision Facts • A more reactive metal will displace a less reactive metal from its compound for example • Magnesium + copper sulphate Magnesium sulphate + copper • Metals below carbon in the reactivity series can be extracted from its ore by heating it with carbon • Ceramic materials are compounds for example silicates and metal oxides • Polymers are long chain molecules. • Wool is an example of a natural polymer. • Polyethene is an example of a synthetic polymer. -

Recording Sheets Containing Amino Acids, Hydroxy Acids, and Polycarboxyl Compounds
Office europeen des brevets (fi) Publication number : 0 667 246 A1 @ EUROPEAN PATENT APPLICATION @ Application number: 95300919.8 @ Int. CI.6: B41M 5/00, D21H 17/14 (22) Date of filing : 14.02.95 (30) Priority : 15.02.94 US 196679 @ Inventor : Malhotra, Shadi L. 4191 Taffey Crescent Mississauga, Ontario LSL2A6 (CA) (43) Date of publication of application : 16.08.95 Bulletin 95/33 (74) Representative : Reynolds, Julian David et al Rank Xerox Ltd @ Designated Contracting States : Patent Department DE FR GB Parkway Marlow Buckinghamshire SL7 1YL (GB) @ Applicant : XEROX CORPORATION Xerox Square Rochester New York 14644 (US) (54) Recording sheets containing amino acids, hydroxy acids, and polycarboxyl compounds. (57) A recording sheet which comprises a paper substrate and a material selected from the group consisting of monomeric amino acids, monomeric hydroxy acids, monomeric polycarboxyl com- pounds, and mixtures thereof. Another embodiment of the present invention is directed to a recording sheet which comprises a substrate and a material selected from the group consisting of monomeric amino acids, monomeric hydroxy acids, and mixtures thereof. CO CM h- (O CO LU Jouve, 18, rue Saint-Denis, 75001 PARIS EP 0 667 246 A1 The present invention is directed to recording sheets, such as transparency materials, filled plastics, pa- pers, and the like. More specifically, the present invention is directed to recording sheets particularly suitable for use in ink jet printing processes. South African Patent Application 924,610 discloses a transparent