Diverse Earliest Triassic Ostracod Fauna of the Non‐
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Volume 2, Chapter 10-2: Arthropods: Crustacea
Glime, J. M. 2017. Arthropods: Crustacea – Ostracoda and Amphipoda. Chapt. 10-2. In: Glime, J. M. Bryophyte Ecology. Volume 2. 10-2-1 Bryological Interaction. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 19 July 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology2/>. CHAPTER 10-2 ARTHROPODS: CRUSTACEA – OSTRACODA AND AMPHPODA TABLE OF CONTENTS CLASS OSTRACODA ..................................................................................................................................... 10-2-2 Adaptations ................................................................................................................................................ 10-2-3 Swimming to Crawling ....................................................................................................................... 10-2-3 Reproduction ....................................................................................................................................... 10-2-3 Habitats ...................................................................................................................................................... 10-2-3 Terrestrial ............................................................................................................................................ 10-2-3 Peat Bogs ............................................................................................................................................ 10-2-4 Aquatic ............................................................................................................................................... -

Crustacea: Ostracoda) in Three Temporary Ponds
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by RERO DOC Digital Library Hydrobiologia (2009) 636:219–232 DOI 10.1007/s10750-009-9952-0 PRIMARY RESEARCH PAPER Dynamics of sexual and parthenogenetic populations of Eucypris virens (Crustacea: Ostracoda) in three temporary ponds Maria Joa˜o Fernandes Martins • Jochen Vandekerkhove • Francesc Mezquita • Olivier Schmit • Juan Rueda • Giampaolo Rossetti • Tadeusz Namiotko Received: 20 May 2009 / Revised: 13 September 2009 / Accepted: 15 September 2009 / Published online: 19 October 2009 Ó Springer Science+Business Media B.V. 2009 Abstract Eucypris virens is a freshwater ostracod This renders the species a potentially valuable in which both sexual reproduction and partheno- model organism to study the ‘queen of evolutionary genesis occur. Sympatric coexistence of both problems’, i.e. why sex is so successful despite its reproductive modes is known in zones of overlap. costs (paradox of sex). In order to maximally exploit this potential, a broad knowledge of the species’ ecology is essential, including an under- standing of its life history and population dynam- ics. Here, the phenology of the species was Electronic supplementary material The online version of followed in three temporary ponds through monthly this article (doi:10.1007/s10750-009-9952-0) contains (Spain) or fortnightly (Poland) samplings, through- supplementary material, which is available to authorized users. out an inundation period. This study confirms the wide ecological tolerances of E. virens. Although Handling editor: K. Martens the species is generally assumed to be univoltine, M. J. F. Martins (&) Á J. Vandekerkhove Á T. Namiotko two hatching periods were observed in the Spanish Laboratory of Limnozoology, Department of Genetics, sites. -

Crustacea, Malacostraca)*
SCI. MAR., 63 (Supl. 1): 261-274 SCIENTIA MARINA 1999 MAGELLAN-ANTARCTIC: ECOSYSTEMS THAT DRIFTED APART. W.E. ARNTZ and C. RÍOS (eds.) On the origin and evolution of Antarctic Peracarida (Crustacea, Malacostraca)* ANGELIKA BRANDT Zoological Institute and Zoological Museum, Martin-Luther-King-Platz 3, D-20146 Hamburg, Germany Dedicated to Jürgen Sieg, who silently died in 1996. He inspired this research with his important account of the zoogeography of the Antarctic Tanaidacea. SUMMARY: The early separation of Gondwana and the subsequent isolation of Antarctica caused a long evolutionary his- tory of its fauna. Both, long environmental stability over millions of years and habitat heterogeneity, due to an abundance of sessile suspension feeders on the continental shelf, favoured evolutionary processes of “preadapted“ taxa, like for exam- ple the Peracarida. This taxon performs brood protection and this might be one of the most important reasons why it is very successful (i.e. abundant and diverse) in most terrestrial and aquatic environments, with some species even occupying deserts. The extinction of many decapod crustaceans in the Cenozoic might have allowed the Peracarida to find and use free ecological niches. Therefore the palaeogeographic, palaeoclimatologic, and palaeo-hydrographic changes since the Palaeocene (at least since about 60 Ma ago) and the evolutionary success of some peracarid taxa (e.g. Amphipoda, Isopo- da) led to the evolution of many endemic species in the Antarctic. Based on a phylogenetic analysis of the Antarctic Tanaidacea, Sieg (1988) demonstrated that the tanaid fauna of the Antarctic is mainly represented by phylogenetically younger taxa, and data from other crustacean taxa led Sieg (1988) to conclude that the recent Antarctic crustacean fauna must be comparatively young. -

The Taxonomy and Biogeography of Macrofaunal Ostracod Crustaceans
The taxonomy and biogeography of macrofaunal ostracod crustaceans, with focus on the abyssal benthic Pacific fauna relevant to the CCFZ Ivana Karanovic Hanyang University, Department of Life Science, College of Natural Sciences, Seoul 133-791, Korea University of Tasmania, IMAS, Hobart, TAS, 7001, Australia e-mail: [email protected] Few words about myself Italy(Salerno, 2 years) Serbia (Novi Sad, born) Australia (Perth & Hobart, 10 years) Germany (Hamburg, 2 years) South Korea (Seoul, 3.5 years) • Started working on ostracods 15 years ago • Worked on faunas from all continents (including Antarctica) • and from all environments: from freshwater puddles to deep sea • I don’t particularly like ostracods • I like the fact that ostracods give insight into many aspects of biology General information on ostracods • Named in 1802 by Latreille • Name comes from the Greek óstrakon, meaning shell or tile • Common name in English: “mussel shrimp” or “seed shrimp” • In German it is “Muschelkrebse” • Live in all aquatic habitats on the planet Fossil record Systematics • Previously in the class Maxillopoda • Currently recognized as one of the 7 classes of the phylum Crustacea Currently divided into two subclasses 1. Myodocopa 2. Podocopa Systematics cont. 4a. 1. Subclass Myodocopa 1. Order Myodocopina 2. Order Halocyprida 4b. a) Suborder Halocypridina b) Suborder Cladocopina 2a. Subclass Podocopa 3. Order Platycopida 4. Order Podocopida 4c. a) Suborder Bairdiocopina b) Suborder Cytherocopina 2b. c) Suborder Darwinulocopina d) Suborder Cypridocopina 4d. e) Suborder Sigilliocopina 3. Photo credits: 4e. 1, 2: S.N. Brandao 3: Brandao & Yasuhara 4b, c: D. Keyser 4e: From Maddocks (1972) Morphology a. -

On Homology of Arthropod Compound Eyes' "The Eye" Has Long Served As
INTEGR. CotoP. BIOL., 43:522-530 (2003) On Homology of Arthropod Compound Eyes' TODD H. OAKLEY 2 Ecology Evolutioni and Marine Biology, University of California-SantaBarbara, S'anzta Barbara, Califonzia 93106 SyNopsis. Eyes serve as models to understand the evolution of complex traits, with broad implications for the origins of evolutionary novelty. Discussions of eye evolution are relevant at miany taxonomic levels, especially within arthropods where compound eye distribution is perplexing. Either compound eyes were lost numerous times or very similar eyes evolved separately in multiple lineages. Arthropod compound eye homology is possible, especially; between crustaceans and hexapods, which have very similar eye facets and may be sister taxa. However, judging homology only on similarity requires subjective decisions. Regardless of whether compound eyes were present in a common ancestor of arthropods or crustaceans + hexapods, recent phylogenetic evidence suggests that the compound eyes, today present in myodocopid ostracods (Crus- tacea), may have been absent in ostracod ancestors. This pattern is inconsistent with phylogenetic homology. Multiple losses of ostracod eyes are an alternative hypothesis that is statistically improbable and without clear cause. One possible evolutionary process to explain the lack of phylogenetic'homology of ostracod compound eyes is that eyes may evolve by switchback evolution, where genes for lost structures remain dormant and are re-expressed much later in evolution. INTRODUCTION 'the recent evidence for and implications of a poten- "The eye" has long served as a canonical example tially non-homologous arthropod compound eye, un- of a complex trait. In an early design-based argument derstanding the case for compound eye homology is for the existence of God, Paley (1846) used the eye as important. -

Ningaloo Coast World Heritage Nomination: (2008)
Ningaloo Coast Ningaloo Coast © Commonwealth of Australia, January 2010 This work is copyright. Apart from any use as permitted under theCopyright Act 1968, no part may be reproduced by any process without prior written permission from the Commonwealth, available from the Australian Government Department of the Environment, Water, Heritage and the Arts. Published by: Department of the Environment, Water, Heritage and the Arts GPO Box 787 Canberra ACT 2061 National Library of Australia Cataloguing-in-Publication data: Commonwealth of Australia Ningaloo Coast: World Heritage nomination I Australia. Dept. of the Environment, Water, Heritage and the Arts. ISBN 978-1-921733-03-1 Designed by 2B Advertising and Design All images © Department of the Environment, Water, Heritage and the Arts (and associated photographers) unless noted. Front cover image: Photograph Tony Howard © Western Australian Department of the Environment and Conservation Ningaloo Coast ❱ F R O M R eef T O R ange Table of ConTenTs ExEcutivE Summary IV KEy tErmS VII PART 1 IDENTIfICaTION OF THe PRoPeRTY 1 1.A COuntry 2 1.B State, province or region 2 1.C Name of property 2 1.D Geographical coordinates 2 1.E Maps and plans, showing the boundaries of the property 3 1.F Area of nominated property 12 PART 2 DESCRIPTION 13 2.A Description of property 14 2.B History and development 44 PART 3 JUsTIfICaTION FOR INSCRIPTION 53 3.A Criteria under which inscription is proposed (and Justification for inscription under these criteria) 54 Criterion (vii) 56 Criterion (viii) 64 Criterion -

Ostracod Assemblages in the Frasassi Caves and Adjacent Sulfidic Spring and Sentino River in the Northeastern Apennines of Italy
D.E. Peterson, K.L. Finger, S. Iepure, S. Mariani, A. Montanari, and T. Namiotko – Ostracod assemblages in the Frasassi Caves and adjacent sulfidic spring and Sentino River in the northeastern Apennines of Italy. Journal of Cave and Karst Studies, v. 75, no. 1, p. 11– 27. DOI: 10.4311/2011PA0230 OSTRACOD ASSEMBLAGES IN THE FRASASSI CAVES AND ADJACENT SULFIDIC SPRING AND SENTINO RIVER IN THE NORTHEASTERN APENNINES OF ITALY DAWN E. PETERSON1,KENNETH L. FINGER1*,SANDA IEPURE2,SANDRO MARIANI3, ALESSANDRO MONTANARI4, AND TADEUSZ NAMIOTKO5 Abstract: Rich, diverse assemblages comprising a total (live + dead) of twenty-one ostracod species belonging to fifteen genera were recovered from phreatic waters of the hypogenic Frasassi Cave system and the adjacent Frasassi sulfidic spring and Sentino River in the Marche region of the northeastern Apennines of Italy. Specimens were recovered from ten sites, eight of which were in the phreatic waters of the cave system and sampled at different times of the year over a period of five years. Approximately 6900 specimens were recovered, the vast majority of which were disarticulated valves; live ostracods were also collected. The most abundant species in the sulfidic spring and Sentino River were Prionocypris zenkeri, Herpetocypris chevreuxi,andCypridopsis vidua, while the phreatic waters of the cave system were dominated by two putatively new stygobitic species of Mixtacandona and Pseudolimnocythere and a species that was also abundant in the sulfidic spring, Fabaeformiscandona ex gr. F. fabaeformis. Pseudocandona ex gr. P. eremita, likely another new stygobitic species, is recorded for the first time in Italy. The relatively high diversity of the ostracod assemblages at Frasassi could be attributed to the heterogeneity of groundwater and associated habitats or to niche partitioning promoted by the creation of a chemoautotrophic ecosystem based on sulfur-oxidizing bacteria. -

A Gigantic Marine Ostracod (Crustacea: Myodocopa) Trapped in Mid-Cretaceous Burmese Amber Received: 16 November 2017 Lida Xing 1,2, Benjamin Sames 3,4, Ryan C
www.nature.com/scientificreports OPEN A gigantic marine ostracod (Crustacea: Myodocopa) trapped in mid-Cretaceous Burmese amber Received: 16 November 2017 Lida Xing 1,2, Benjamin Sames 3,4, Ryan C. McKellar5,6, Dangpeng Xi1,2, Ming Bai7 & Accepted: 9 January 2018 Xiaoqiao Wan1,2 Published: xx xx xxxx The mid-Cretaceous Burmese amber (~99 Ma, Myanmar), widely known for exquisite preservation of theropods, also yields microfossils, which can provide important contextual information on paleoenvironment and amber formation. We report the frst Cretaceous ostracod in amber—the gigantic (12.9 mm) right valve of an exclusively marine group (Myodocopa: Myodocopida) preserved in Burmese amber. Ostracods are usually small (0.5–2 mm), with well-calcifed carapaces that provide an excellent fossil record extending to at least the Ordovician (~485 million years ago), but they are rarely encountered in amber. The new specimen efectively doubles the age of the ostracod amber record, ofering the frst representative of the Myodocopa, a weakly calcifed group with a poor fossil record. Its carapace morphology is atypical and likely plesiomorphic. The preserved valve appears to be either a moulted exuvium or a dead and disarticulated specimen, and subsequent resin fows contain forest foor inclusions with terrestrial arthropods, i.e., fragmentary remains of spiders, and insect frass. These features resolve an enigmatic taphonomic pathway, and support a marginal marine setting for resin production. Ostracods are aquatic microcrustaceans, with a calcareous, bivalved shell (carapace) that can enclose the whole body and all appendages. Few Mesozoic to Recent taxa exceed 3 mm in size and these are termed ‘gigantic’ ostra- cods, such as species of the living marine planktonic genus Gigantocypris (subclass Myodocopa, up to around 30 mm), or of the non-marine genus Megalocypris (subclass Podocopa, 5–8 mm in size). -

Palynology, Microfacies and Ostracods of the Permian–Triassic Boundary Interval in the Rosengarten/Catinaccio Massif (Southern Alps, Italy)
Austrian Journal of Earth Sciences Vienna 2019 Volume 112/2 103 - 124 DOI: 10.17738/ajes.2019.0007 Palynology, microfacies and ostracods of the Permian–Triassic boundary interval in the Rosengarten/Catinaccio Massif (Southern Alps, Italy) Hendrik NOWAK1)*, Wolfgang METTE2), Fabio M. PETTI3), Guido ROGHI4), Evelyn KUSTATSCHER1),5),6) 1) Museum of Nature South Tyrol, Bindergasse/Via Bottai 1, 39100 Bozen/Bolzano, Italy; e-mail: [email protected]; evelyn.kustatscher@ naturmuseum.it 2) Department of Geology, Universität Innsbruck, Innrain 52f, 6020 Innsbruck, Austria; e-mail: [email protected] 3) MUSE – Museo delle Scienze di Trento, Corso del Lavoro e della Scienza 3, Trento 38122, Italy; e-mail: [email protected] 4) Istituto di Geoscienze e Georisorse - CNR, Via Gradenigo 6, Padova 35131, Italy; e-mail: [email protected] 5) Department of Earth and Environmental Sciences, Paleontology & Geobiology, Ludwig-Maximilians-Universität München, Richard-Wagner-Straße 10, 80333 München, Germany 6) SNSB-Bayerische Staatssammlung für Paläontologie und Geologie, Richard-Wagner-Straße 10, 80333 München, Germany *) Corresponding author: Hendrik NOWAK KEYWORDS upper Permian, Lopingian, Lower Triassic, Dolomites, Bellerophon Formation, Werfen Formation. Abstract The Laurinswand section in the Rosengarten/Catinaccio Massif (Dolomites, Southern Alps, Italy) covers the Permian– Triassic boundary in a proximal marine setting. The section has been studied for palynology, ostracods and carbonate microfacies. Five microfacies types are defined for the carbonates of the Bellerophon Formation (Changhsingian) in this section. Ostracod assemblages from the upper Bellerophon Formation show a moderate to high diversity and mostly indi- cate normal marine conditions, with some samples from the upper Casera Razzo Member being dominated by eurytopic forms. -
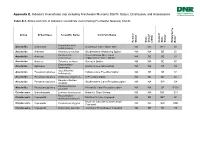
Status, Distribution, and Associations
Appendix E. Indiana’s Invertebrate (not including Freshwater Mussels) SGCN: Status, Distribution, and Associations Table E-1. Status and rank of Indiana’s invertebrate (not including Freshwater Mussels) SGCN. 1 2 2 Group Order/Class Scientific Name Common Name 3 Federal Status State Status (2005) Current State Status NatureServe Rank Hamohalacarus Arachnids Actinedida Donaldson Cave Water Mite NA NA SE* S1 subterraneus Arachnids Araneae Anahita punctulata Southeastern Wandering Spider NA NA SE S1 Porrhomma Cavernicolous Sheet-web Arachnids Araneae NA SE SE S1 cavernicola (Appalachian Cave) Spider Arachnids Araneae Talanites echinus Sac-web Spider NA NA SE S1 Erebomaster Arachnids Opiliones Golden Cave Harvestman NA NA ST S2 flavescens Apochthonius Arachnids Pseudoscorpiones Indiana Cave Pseudoscorpion NA SE SE S1 indianensis Arachnids Pseudoscorpiones Chthonius virginicus A Pseudoscorpion NA SE SE S1 Hesperochernes Arachnids Pseudoscorpiones Southeastern Cave Pseudoscorpion NA NA SW S4 mirabilis Kleptochthonius Arachnids Pseudoscorpiones Packard's Cave Pseudoscorpion NA NA SE S1S2 packardi Crustaceans Branchiopoda Lynceus brachyurus Holarctic Clam Shrimp NA NA SW* S1? Bryocamptus Crustaceans Copepoda Morrison's Cave Copepod NA SE SE* S1 morrisoni morrisoni Northern Cavefish (Commensal) Crustaceans Copepoda Cauloxenus stygius NA NA SW SNR Copepod Crustaceans Copepoda Diacyclops jeanneli Jeannel's Groundwater Copepod NA SE ST S2 1 2 2 Group Order/Class Scientific Name Common Name 3 Federal Status State Status (2005) Current State Status NatureServe Rank Megacyclops Crustaceans Copepoda Donaldson's Cave Copepod NA SE SE S1 donnaldsoni Crustaceans Malacostraca Caecidotea jordani Jordan's Groundwater Isopod NA SE SE S1 Crustaceans Malacostraca Caecidotea rotunda Northeastern (Frost) Cave Isopod NA SE SR S3 Indiana University Southeast Crustaceans Malacostraca Caecidotea teresae NA SE SE S1 Groundwater Isopod Crustaceans Malacostraca Crangonyx packardi Packard's Groundwater Amphipod NA SC SW S4 Crustaceans Malacostraca Crangonyx sp. -

The Genome Sizes of Ostracod Crustaceans Correlate with Body Size and Phylogeny 2 3 Nicholas W
bioRxiv preprint doi: https://doi.org/10.1101/114660; this version posted March 7, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 The genome sizes of ostracod crustaceans correlate with body size and phylogeny 2 3 Nicholas W. Jeffery1*, Emily A. Ellis2, Todd H. Oakley2, T. Ryan Gregory1 4 5 *Present address: Fisheries and Oceans Canada, Bedford Institute of Oceanography, Dartmouth, 6 Nova Scotia 7 8 1Department of Integrative Biology, University of Guelph, Guelph, Ontario, Canada. N1G 2W1 9 2University of California Santa Barbara, Santa Barbara, California, USA. 93106 10 11 Corresponding author email: [email protected] 12 13 14 Keywords: Ostracod, genome size, C-value, body size, phylogeny 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 bioRxiv preprint doi: https://doi.org/10.1101/114660; this version posted March 7, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 46 Abstract 47 48 Within animals a positive correlation between genome size and body size has been detected in 49 several taxa but not in others, such that it remains unknown how pervasive this pattern may be. -

Multi-Level Convergence of Complex Traits and the Evolution of Bioluminescence Emily S
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 15 December 2020 Multi-level convergence of complex traits and the evolution of bioluminescence Emily S. Lau* and Todd H. Oakley* Department of Ecology, Evolution, and Marine Biology, University of California Santa Barbara, Santa Barbara CA 93106 Corresponding authors information: Emily S. Lau: [email protected]; Todd H. Oakley: [email protected] Keywords multi‐level convergence | evolution | bioluminescence | biological organization | complex trait ABSTRACT Evolutionary convergence provides natural opportunities to investigate how, when, and why novel traits evolve. Many convergent traits are complex, highlighting the importance of explicitly considering convergence at different levels of biological organization, or ‘multi‐level convergent evolution’. To investigate multi‐level convergent evolution, we propose a holistic and hierarchical framework that emphasizes breaking down traits into several functional modules. We begin by identifying long‐standing questions on the origins of complexity and the diverse evolutionary processes underlying phenotypic convergence to discuss how they can be addressed by examining convergent systems. We argue that bioluminescence, a complex trait that evolved dozens of times through either novel mechanisms or conserved toolkits, is particularly well suited for these studies. We present an updated estimate of at least 94 independent origins of bioluminescence across the tree of life, which we calculated by reviewing and summarizing all estimates of independent origins. Then, we use our framework to review the biology, chemistry, and evolution of bioluminescence, and for each biological level identify questions that arise from our systematic review. We focus on luminous organisms that use the shared luciferin substrates coelenterazine or vargulin to produce light because these organisms convergently evolved bioluminescent proteins that use the same luciferins to produce bioluminescence.