Product Characteristics Chart | Gvoke™ (Glucagon Injection)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endocrine Pathology (537-577)
LABORATORY INVESTIGATION THE BASIC AND TRANSLATIONAL PATHOLOGY RESEARCH JOURNAL LI VOLUME 99 | SUPPLEMENT 1 | MARCH 2019 2019 ABSTRACTS ENDOCRINE PATHOLOGY (537-577) MARCH 16-21, 2019 PLATF OR M & 2 01 9 ABSTRACTS P OSTER PRESENTATI ONS EDUCATI ON C O M MITTEE Jason L. Hornick , C h air Ja mes R. Cook R h o n d a K. Y a nti s s, Chair, Abstract Revie w Board S ar a h M. Dr y and Assign ment Co m mittee Willi a m C. F a q ui n Laura W. La mps , Chair, C ME Subco m mittee C ar ol F. F ar v er St e v e n D. Billi n g s , Interactive Microscopy Subco m mittee Y uri F e d ori w Shree G. Shar ma , Infor matics Subco m mittee Meera R. Ha meed R aj a R. S e et h al a , Short Course Coordinator Mi c h ell e S. Hir s c h Il a n W ei nr e b , Subco m mittee for Unique Live Course Offerings Laksh mi Priya Kunju D a vi d B. K a mi n s k y ( Ex- Of ici o) A n n a M ari e M ulli g a n Aleodor ( Doru) Andea Ri s h P ai Zubair Baloch Vi nita Parkas h Olca Bast urk A nil P ar w a ni Gregory R. Bean , Pat h ol o gist-i n- Trai ni n g D e e p a P atil D a ni el J. -

Adrenal Neuroblastoma Mimicking Pheochromocytoma in an Adult With
Khalayleh et al. Int Arch Endocrinol Clin Res 2017, 3:008 Volume 3 | Issue 1 International Archives of Endocrinology Clinical Research Case Report : Open Access Adrenal Neuroblastoma Mimicking Pheochromocytoma in an Adult with Neurofibromatosis Type 1 Harbi Khalayleh1, Hilla Knobler2, Vitaly Medvedovsky2, Edit Feldberg3, Judith Diment3, Lena Pinkas4, Guennadi Kouniavsky1 and Taiba Zornitzki2* 1Department of Surgery, Hebrew University Medical School of Jerusalem, Israel 2Endocrinology, Diabetes and Metabolism Institute, Kaplan Medical Center, Hebrew University Medical School of Jerusalem, Israel 3Pathology Institute, Kaplan Medical Center, Israel 4Nuclear Medicine Institute, Kaplan Medical Center, Israel *Corresponding author: Taiba Zornitzki, MD, Endocrinology, Diabetes and Metabolism Institute, Kaplan Medical Center, Hebrew University Medical School of Jerusalem, Bilu 1, 76100 Rehovot, Israel, Tel: +972-894- 41315, Fax: +972-8 944-1912, E-mail: [email protected] Context 2. This is the first reported case of an adrenal neuroblastoma occurring in an adult patient with NF1 presenting as a large Neurofibromatosis type 1 (NF1) is a genetic disorder asso- adrenal mass with increased catecholamine levels mimicking ciated with an increased risk of malignant disorders. Adrenal a pheochromocytoma. neuroblastoma is considered an extremely rare tumor in adults and was not previously described in association with NF1. 3. This case demonstrates the clinical overlap between pheo- Case description: A 42-year-old normotensive woman with chromocytoma and neuroblastoma. typical signs of NF1 underwent evaluation for abdominal pain, Keywords and a large 14 × 10 × 16 cm left adrenal mass displacing the Adrenal neuroblastoma, Neurofibromatosis type 1, Pheo- spleen, pancreas and colon was found. An initial diagnosis of chromocytoma, Neural crest-derived tumors pheochromocytoma was done based on the known strong association between pheochromocytoma, NF1 and increased catecholamine levels. -

Ganglioneuroblastoma As Vasoactive Intestinal Polypeptide-Secreting10.5005/Jp-Journals-10002-1167 Tumor: Rare Case Report in a Child Case Report
WJOES Ganglioneuroblastoma as Vasoactive Intestinal Polypeptide-secreting10.5005/jp-journals-10002-1167 Tumor: Rare Case Report in a Child CASE REPORT Ganglioneuroblastoma as Vasoactive Intestinal Polypeptide-secreting Tumor: Rare Case Report in a Child 1Basant Kumar, 2Vijai D Upadhyaya, 3Ram Nawal Rao, 4Sheo Kumar ABSTRACT than 60 cases of pediatric VIP-secreting tumors.3 Most Pathologically elevated vasoactive intestinal polypeptide (VIP) of them are either adrenal pheochromocytoma or mixed plasma levels cause secretory diarrhea with excessive loss of pheochromocytoma-ganglioneuroma tumors. Mason water and electrolyte and is characterized by the typical symp- et al,4 first described the secretory nature of neuroblas- toms of hypokalemia and metabolic acidosis. It rarely occurs toma and vasoactive intestinal peptide (VIP) can be in patients with non-pancreatic disease. Despite the clinical severity, diagnosis of a VIP-secreting tumor is often delayed. produced by the mature neurogenic tumors. We herein We herein present a 14-month-old boy having prolonged present a 14 months old boy having prolonged therapy- therapy-resistant secretory diarrhea, persistent hypokalemia resistant secretory diarrhea, persistent hypokalemia with with tissue diagnosis of ganglioneuroblastoma and raised plasma VIP-levels. tissue diagnosis of ganglioneuroblastoma and briefly review the literature. Keywords: Ganglioneuroblastoma, Secretory diarrhea, VIPoma. CASE REPORT How to cite this article: Kumar B, Upadhyaya VD, Rao RN, Kumar S. Ganglioneuroblastoma as Vasoactive Intestinal A 14-month-old boy, weighing 9 kg with advanced symp- Polypeptide-secreting Tumor: Rare Case Report in a Child. World J Endoc Surg 2015;7(2):47-50. toms of persistent secretory diarrhea, hypokalemia and metabolic acidosis was referred to us with radiological Source of support: Nil (computed tomography) diagnosis of retroperitoneal Conflict of interest: None mass. -

Small Extra-Adrenal Pheochromocytoma Causing Severe Hypertension in an Elderly Patient
635 Hypertens Res Vol.29 (2006) No.8 p.635-638 Case Report Small Extra-Adrenal Pheochromocytoma Causing Severe Hypertension in an Elderly Patient Einosuke MIZUTA1), Toshihiro HAMADA2), Shin-ichi TANIGUCHI2), Masaki SHIMOYAMA2), Takahiro NAWADA3), Junichiro MIAKE2), Yasuhiro KAETSU2), Li PEILI2), Kiyosuke ISHIGURO4), Shingo ISHIGURO4), Osamu IGAWA2), Chiaki SHIGEMASA2), and Ichiro HISATOME1) We report the case of a 67-year-old woman with severe hypertension caused by an extra-adrenal pheochro- mocytoma. The tumor was detected by 131I metaiodobenzylguanidine scintigraphy and it was found to be small (2 cm ø) by enhanced CT. After the extirpation of the tumor, the blood pressure of the patient imme- diately normalized. It should be taken into account that a small extra-adrenal pheochromocytoma can be one of the causes of secondary hypertension in elderly patients. Since small extra-adrenal pheochromocytomas are difficult to detect, it is also important to perform suitable examinations to establish the diagnosis. Fur- thermore, we emphasize the importance of an accurate diagnosis in elderly patients with pheochromocy- toma, for they often have less symptomatology and more severe cardiovascular complications due to refractory hypertension than younger patients. (Hypertens Res 2006; 29: 635–638) Key Words: pheochromocytoma, extra-adrenal, hypertension, diagnosis mately 30% of these patients do not present these signs (7). Introduction Since most of their clinical signs and symptoms are derived from the actions of catecholamines secreted from the adrenal Pheochromocytoma is one of the major causes of secondary glands, adrenal pheochromocytoma induces more severe clin- hypertension, drug-resistant hypertension, and malignant ical signs than those observed in extra-adrenal pheochro- hypertension (1–3), but the rate of occurrence of the tumor mocytoma (7). -

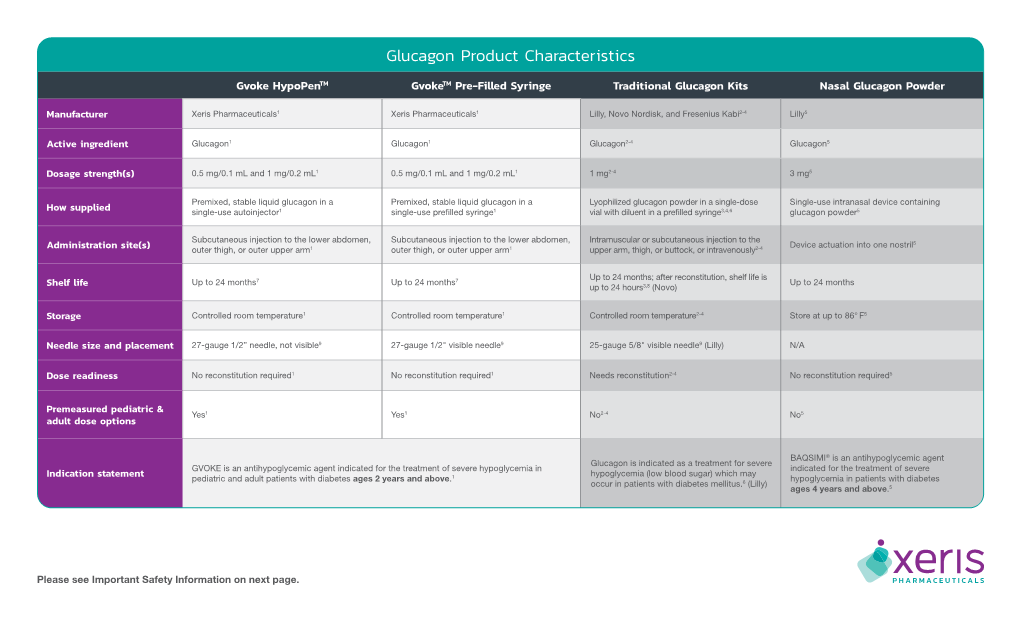
(Glucagon) – New Drug Approval
Baqsimi™ (glucagon) – New drug approval • On July 24, 2019, the FDA announced the approval of Eli Lilly’s Baqsimi (glucagon) nasal powder, for the treatment of severe hypoglycemia in patients with diabetes ages 4 years and above. • Severe hypoglycemia occurs when a patient’s blood sugar levels fall to a level where he or she becomes confused or unconscious or suffers from other symptoms that require assistance from another person to treat. Typically, severe hypoglycemia occurs in people with diabetes who are using insulin treatment. — Injectable glucagon for the treatment of hypoglycemia has been approved for use in the U.S. for several decades. • Baqsimi is the first nasally administered formulation of glucagon. It is ready to use with no reconstitution required. • The efficacy of Baqsimi was evaluated in an open-label, crossover study in 70 adult patients with type 1 diabetes. The efficacy of a single 3 mg dose of Baqsimi was compared to a 1 mg dose of intramuscular glucagon (IMG). The primary efficacy measure was the proportion of patients achieving treatment success, which was defined as either an increase in blood glucose to ≥ 70 mg/dL or an increase of ≥ 20 mg/dL from glucose nadir within 30 minutes after receiving glucagon, without receiving additional actions to increase the blood glucose level. — Baqsimi demonstrated non-inferiority to IMG in reversing insulin-induced hypoglycemia with 100% of Baqsimi-treated patients and 100% of IMG-treated patients achieving treatment success. • The efficacy of Baqsimi was also evaluated in a similarly designed study in 83 adult patients with type 1 or type 2 diabetes. -

Multiple Endocrine Neoplasia Type 2: an Overview Jessica Moline, MS1, and Charis Eng, MD, Phd1,2,3,4
GENETEST REVIEW Genetics in Medicine Multiple endocrine neoplasia type 2: An overview Jessica Moline, MS1, and Charis Eng, MD, PhD1,2,3,4 TABLE OF CONTENTS Clinical Description of MEN 2 .......................................................................755 Surveillance...................................................................................................760 Multiple endocrine neoplasia type 2A (OMIM# 171400) ....................756 Medullary thyroid carcinoma ................................................................760 Familial medullary thyroid carcinoma (OMIM# 155240).....................756 Pheochromocytoma ................................................................................760 Multiple endocrine neoplasia type 2B (OMIM# 162300) ....................756 Parathyroid adenoma or hyperplasia ...................................................761 Diagnosis and testing......................................................................................756 Hypoparathyroidism................................................................................761 Clinical diagnosis: MEN 2A........................................................................756 Agents/circumstances to avoid .................................................................761 Clinical diagnosis: FMTC ............................................................................756 Testing of relatives at risk...........................................................................761 Clinical diagnosis: MEN 2B ........................................................................756 -

Pheochromocytoma of the Pancreas: a Report of Three Cases and a Literature Review
ONCOLOGY LETTERS 12: 959-962, 2016 Pheochromocytoma of the pancreas: A report of three cases and a literature review MIN YANG1*, HUI DING2*, MIN CAI2, YAN-AN HE2, YU CAI2, YONG ZENG2 and BO-LE TIAN1 1Department of Pancreatic Surgery, West China Hospital of Sichuan University, Chengdu, Sichuan 610041; 2Department of Hepatobiliary Surgery, People's Hospital of Jiangyou, Mianyang, Sichuan 621700, P.R. China Received December 14, 2014; Accepted December 11, 2015 DOI: 10.3892/ol.2016.4721 Abstract. Pheochromocytoma is primarily derived from the Introduction adrenal medulla. The majority of extra-adrenal pheochro- mocytoma cases occur in the superior para-aortic region and Pheochromocytoma is a catecholamine-secreting tumor that para-adrenal area. However, pheochromocytoma originating originates from chromaffin cells. The majority of these tumors from the pancreas is rare. The present study reports the cases arise in the adrenal medulla (1), and when they occur outside of three patients who had no history of hypertension but were the adrenal gland they are known as an extra-adrenal pheo- post-operatively diagnosed with pheochromocytoma located chromocytoma or a paraganglioma (2). Pheochromocytoma in the pancreas. Of the three patients, two were admitted is primarily sporadic, but may additionally be associated to hospital due to abdominal pain, and imaging examina- with multiple endocrine neoplasia syndrome (3) and muta- tions revealed a soft-tissue lesion in the head of pancreas. tions of mitochondrial complex 2 succinate dehydrogenase Local resection of the pancreatic tumor was successfully enzymes (4). performed and a diagnosis of pheochromocytoma derived Extra-adrenal pheochromocytoma has been previously from the pancreas was subsequently made by pathologists. -

Adrenal Cortical Tumors, Pheochromocytomas and Paragangliomas
Modern Pathology (2011) 24, S58–S65 S58 & 2011 USCAP, Inc. All rights reserved 0893-3952/11 $32.00 Adrenal cortical tumors, pheochromocytomas and paragangliomas Ricardo V Lloyd Department of Pathology, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA Distinguishing adrenal cortical adenomas from carcinomas may be a difficult diagnostic problem. The criteria of Weiss are very useful because of their reliance on histologic features. From a practical perspective, the most useful criteria to separate adenomas from carcinomas include tumor size, presence of necrosis and mitotic activity including atypical mitoses. Adrenal cortical neoplasms in pediatric patients are more difficult to diagnose and to separate adenomas from carcinomas. The diagnosis of pediatric adrenal cortical carcinoma requires a higher tumor weight, larger tumor size and more mitoses compared with carcinomas in adults. Pheochromocytomas are chromaffin-derived tumors that develop in the adrenal gland. Paragangliomas are tumors arising from paraganglia that are distributed along the parasympathetic nerves and sympathetic chain. Positive staining for chromogranin and synaptophysin is present in the chief cells, whereas the sustentacular cells are positive for S100 protein. Hereditary conditions associated with pheochromocytomas include multiple endocrine neoplasia 2A and 2B, Von Hippel–Lindau disease and neurofibromatosis I. Hereditary paraganglioma syndromes with mutations of SDHB, SDHC and SDHD are associated with paragangliomas and some pheochromocytomas. -

Pancreatic Neuroendocrine Tumours in Patients with Von Hippel-Lindau Disease
Review Endokrynologia Polska DOI: 10.5603/EP.a2020.0027 Volume/Tom 71; Number/Numer 3/2020 ISSN 0423–104X Pancreatic neuroendocrine tumours in patients with von Hippel-Lindau disease Agnieszka Zwolak1, 2, Joanna Świrska1, 2, Ewa Tywanek1, 2, Marta Dudzińska1, Jerzy S. Tarach2, Beata Matyjaszek-Matuszek2 1Chair of Internal Medicine and Department of Internal Nursing, Medical University in Lublin, Poland 2Department of Endocrinology, Medical University in Lublin, Poland Abstract Von Hippel-Lindau disease is a highly penetrant autosomal genetic disorder caused by a germline mutation in the tumour suppressor gene, manifesting with the formation of various tumours, including neuroendocrine tumours of the pancreas. The incidence of the latter is not very high, varying from 5% to 18%. To compare, haemangioblastomas and clear cell renal carcinoma are present in 70% of von Hippel-Lindau patients and are considered the main prognostic factors, with renal cancer being the most common cause of death. However, pancreatic neuroendocrine tumours should not be neglected, considering their malignant potential (different to sporadic cases), natural history, and treatment protocol. This paper aims to review the literature on the epidemiology, natural history, treatment, and surveillance of individuals affected by pancreatic neuroendocrine tumours in von Hippel-Lindau disease. (Endokrynol Pol 2020; 71 (3): 256–259) Key words: pancreatic neuroendocrine tumours; von Hippel-Lindau disease REVIEW Introduction 52 VHL patients evaluated by Hough, there were six people (12%) in whom pancreatic involvement was the Von Hippel-Lindau disease (VHL) is a highly penetrant only abdominal manifestation of the disease [9]. Fur- autosomal genetic disorder caused by a germline muta- thermore, in the study by Hammel, VHL disease was tion in the VHL tumour suppressor gene located on the diagnosed accidently in 6% of patients during imaging short arm of chromosome 3. -

Series of Rare Diagnosis and Presentations of Abdominal Mass in Children: a Great Learning Experience
International Journal of Contemporary Pediatrics Ghosh A et al. Int J Contemp Pediatr. 2020 May;7(5):1184-1190 http://www.ijpediatrics.com pISSN 2349-3283 | eISSN 2349-3291 DOI: http://dx.doi.org/10.18203 /2349-3291.ijcp20201658 Case report Series of rare diagnosis and presentations of abdominal mass in children: a great learning experience Arindam Ghosh, Subhankar Chakravorty, Somak Krishna Biswas*, Sumitra Kumar Biswas Department of Pediatric Surgery, N. R. S. Medical College and Hospital, Kolkata, West Bengal, India Received: 18 February 2020 Accepted: 27 March 2020 *Correspondence: Dr. Somak Krishna Biswas, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Abdominal mass is a common finding in children, either palpable or radiologically evident surprisingly. Some of them are rare tumors. Functional active tumors are rarely palpable but present with varied arrays of symptoms. In this series authors are discussing few rare cases with atypical presentations such as- teratoma arising from adrenal gland, teratoma presenting with hypertension, fetus in fetu (Girl and Boy child), adrenocortical tumor presenting as precocious puberty and adrenal pheochromocytoma with features of cushing’s syndrome. These atypical presentations may have pose a challenge in diagnosis and management for the treating team with first time occurrence specifically if they are handling them for first time. Keywords: Abdominal mass in children, Adrenal tumor, Fetus-in-fetu, Teratoma INTRODUCTION associated symptoms. -

A Catecholamine-Secreting Neuroblastoma Leading to Hydrops Fetalis
Journal of Perinatology (2014) 34, 405–407 & 2014 Nature America, Inc. All rights reserved 0743-8346/14 www.nature.com/jp PERINATAL/NEONATAL CASE PRESENTATION A catecholamine-secreting neuroblastoma leading to hydrops fetalis T Inoue1,YIto1, T Nakamura1, K Matsuoka2 and H Sago1 A case of fetal neuroblastoma of the right adrenal gland, with rapid development of hydrops fetalis due to catecholamine-induced cardiomyopathy, is reported. A fetus with a right suprarenal mass detected during ultrasonography at 32 weeks gestation progressively developed into hydrops fetalis by 35.2 weeks gestation. An emergent cesarean section was performed. At birth, the female neonate was hypertensive, with markedly elevated catecholamine levels; echocardiography showed poor contractility. Morphine, human atrial natriuretic peptide, milrinone, nitroprusside and dobutamine were initiated and her blood pressure was maintained within the normal range and her cardiac contractility improved 2 weeks after birth. Neuroblastoma cells were detected in the placenta, resulting in the right adrenal mass being diagnosed as a neuroblastoma. She was well, and the mass diminished in size within 4 months, without surgery. A fetus with suspected neuroblastoma, indicated by a suprarenal mass, should be managed with appropriate consideration of hydrops. Journal of Perinatology (2014) 34, 405–407; doi:10.1038/jp.2014.19 Keywords: congenital neuroblastoma; catecholamine-induced cardiomyopathy; placental metastasis; paroxysmal hypertension INTRODUCTION Upon delivery, the female neonate had generalized edema and Neuroblastomas constitute 25% of congenital malignancies,1 and weighed 3268 g, with 1 and 5 min Apgar scores of 8; she was in some secrete catecholamine, causing hypertension. Catechola- respiratory distress, necessitating mechanical ventilation. The baby mine excess can cause oxygen free radical development, calcium was hypertensive, with a blood pressure (BP) of 76/47/59 mm Hg overload in the heart, downregulation of cardiac b-adrenergic (systolic/diastolic/mean). -

Evolving the Paradigm in Severe Hypoglycemia Management
PRESCRIBING INFORMATION | IMPORTANT SAFETY INFORMATION YOU ARE INVITED TO A NATIONAL WEBINAR EVENT Evolving the Paradigm in Severe Hypoglycemia Management SPEAKER: Davida F. Kruger, MSN, APN-BC, BC-ADM Certified Nurse Practitioner Henry Ford Health System Detroit, MI WEBINAR DATE: Wednesday, February 24, 2021 6:30 PM ET TO REGISTER, VISIT: www.HypoglycemiaManagement.com Meeting Objectives • Consider the serious issue of severe • Recognize administration challenges of hypoglycemia in diabetes, including its existing emergency glucagon kits prevalence and impact on affected individuals • Integrate the insights from today’s and their glycemic management presentation into an action plan for the • Discuss how emerging standards of care for identification and management of patients severe hypoglycemia can help overcome the at risk of severe hypoglycemia limitations of earlier guidelines Please join us for a virtual event on Evolving the Paradigm in Severe Hypoglycemia Management. During this webinar we will focus on the objectives outlined above. This event is not eligible for CME credit. This event complies with PhRMA guidelines. Attendance is limited to healthcare professionals. Questions: Please contact POCN at [email protected] IMPORTANT SAFETY INFORMATION GVOKE is indicated for the treatment of severe hypoglycemia in adult and pediatric patients with diabetes ages 2 years and above. Contraindications GVOKE is effective in treating hypoglycemia only GVOKE is contraindicated in patients with if sufficient hepatic glycogen is present. Patients pheochromocytoma, insulinoma, and known in states of starvation, with adrenal insufficiency hypersensitivity to glucagon or to any of the or chronic hypoglycemia, may not have excipients in GVOKE. Allergic reactions have been adequate levels of hepatic glycogen for GVOKE reported with glucagon and include anaphylactic administration to be effective.