Specifying Neurons and Circuits for Limb Motor Control
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Genetic Analysis of Retinopathy in Type 1 Diabetes
Genetic Analysis of Retinopathy in Type 1 Diabetes by Sayed Mohsen Hosseini A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Institute of Medical Science University of Toronto © Copyright by S. Mohsen Hosseini 2014 Genetic Analysis of Retinopathy in Type 1 Diabetes Sayed Mohsen Hosseini Doctor of Philosophy Institute of Medical Science University of Toronto 2014 Abstract Diabetic retinopathy (DR) is a leading cause of blindness worldwide. Several lines of evidence suggest a genetic contribution to the risk of DR; however, no genetic variant has shown convincing association with DR in genome-wide association studies (GWAS). To identify common polymorphisms associated with DR, meta-GWAS were performed in three type 1 diabetes cohorts of White subjects: Diabetes Complications and Control Trial (DCCT, n=1304), Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR, n=603) and Renin-Angiotensin System Study (RASS, n=239). Severe (SDR) and mild (MDR) retinopathy outcomes were defined based on repeated fundus photographs in each study graded for retinopathy severity on the Early Treatment Diabetic Retinopathy Study (ETDRS) scale. Multivariable models accounted for glycemia (measured by A1C), diabetes duration and other relevant covariates in the association analyses of additive genotypes with SDR and MDR. Fixed-effects meta- analysis was used to combine the results of GWAS performed separately in WESDR, ii RASS and subgroups of DCCT, defined by cohort and treatment group. Top association signals were prioritized for replication, based on previous supporting knowledge from the literature, followed by replication in three independent white T1D studies: Genesis-GeneDiab (n=502), Steno (n=936) and FinnDiane (n=2194). -

Your Inner Fish
CHAPTER THREE HANDY GENES While my colleagues and I were digging up the first Tiktaalik in the Arctic in July 2004, Randy Dahn, a researcher in my laboratory, was sweating it out on the South Side of Chicago doing genetic experiments on the embryos of sharks and skates, cousins of stingrays. You’ve probably seen small black egg cases, known as mermaid’s purses, on the beach. Inside the purse once lay an egg with yolk, which developed into an embryonic skate or ray. Over the years, Randy has spent hundreds of hours experimenting with the embryos inside these egg cases, often working well past midnight. During the fateful summer of 2004, Randy was taking these cases and injecting a molecular version of vitamin A into the eggs. After that he would let the eggs develop for several months until they hatched. His experiments may seem to be a bizarre way to spend the better part of a year, let alone for a young scientist to launch a promising scientific career. Why sharks? Why a form of vitamin A? 61 To make sense of these experiments, we need to step back and look at what we hope they might explain. What we are really getting at in this chapter is the recipe, written in our DNA, that builds our bodies from a single egg. When sperm fertilizes an egg, that fertilized egg does not contain a tiny hand, for instance. The hand is built from the information contained in that single cell. This takes us to a very profound problem. -

Individual Neuronal Subtypes Control Initial Myelin Sheath Growth and Stabilization Heather N
Nelson et al. Neural Development (2020) 15:12 https://doi.org/10.1186/s13064-020-00149-3 RESEARCH ARTICLE Open Access Individual neuronal subtypes control initial myelin sheath growth and stabilization Heather N. Nelson, Anthony J. Treichel, Erin N. Eggum, Madeline R. Martell, Amanda J. Kaiser, Allie G. Trudel, James R. Gronseth, Samantha T. Maas, Silas Bergen and Jacob H. Hines* Abstract Background: In the developing central nervous system, pre-myelinating oligodendrocytes sample candidate nerve axons by extending and retracting process extensions. Some contacts stabilize, leading to the initiation of axon wrapping, nascent myelin sheath formation, concentric wrapping and sheath elongation, and sheath stabilization or pruning by oligodendrocytes. Although axonal signals influence the overall process of myelination, the precise oligodendrocyte behaviors that require signaling from axons are not completely understood. In this study, we investigated whether oligodendrocyte behaviors during the early events of myelination are mediated by an oligodendrocyte-intrinsic myelination program or are over-ridden by axonal factors. Methods: To address this, we utilized in vivo time-lapse imaging in embryonic and larval zebrafish spinal cord during the initial hours and days of axon wrapping and myelination. Transgenic reporter lines marked individual axon subtypes or oligodendrocyte membranes. Results: In the larval zebrafish spinal cord, individual axon subtypes supported distinct nascent sheath growth rates and stabilization frequencies. Oligodendrocytes ensheathed individual axon subtypes at different rates during a two-day period after initial axon wrapping. When descending reticulospinal axons were ablated, local spinal axons supported a constant ensheathment rate despite the increased ratio of oligodendrocytes to target axons. Conclusion: We conclude that properties of individual axon subtypes instruct oligodendrocyte behaviors during initial stages of myelination by differentially controlling nascent sheath growth and stabilization. -

Whole-Organism Imaging of Spinal Motor Neurons and Musculature with the Cytation C10 Confocal Imaging Reader
Application Bulletin Whole-Organism Imaging of Spinal Motor Neurons and Musculature with the Cytation C10 Confocal Imaging Reader Introduction Zebrafish have long held an essential oler in developmental biology research due to their many and often cited advantages, both biological and practical. Recently, zebrafish amenability to genome editing techniques such as CRISPR, high-throughput screening, and predictive value in drug discovery toxicol- ogy studies1, combined with their exceptional accessibility to optogenetic manipulation and live-imaging techniques highlight the essential place zebrafish have in the toolkit of health-driven research. Indeed, zebrafish are lowering the barrier toin vivo research of devastating neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS, also known as Lou Gehrig’s disease)2, as their complete spinal neuron and muscle unit organization is entirely accessible to observation through light microscopy. This application bulletin demonstrates the capability of whole-organism imaging on the newly intro- duced BioTek Cytation C10 confocal imaging reader, with a focus on spinal motor neurons, neuromus- cular synapses, and musculature of the zebrafish model organism. The optical sectioning of the spin- ning disk confocal system is essential to resolving the deep tissue structures, such as the fine neuronal processes, and provides a literal clearer view of subcellular details when imaging thick samples. Materials and Methods Key Words: Unless otherwise noted, all chemicals were obtained from Sigma Aldrich (St. Louis, MO). General main- tenance of zebrafish followed established methods3. Wild-type male and female Danio rerio were main- Confocal Microscopy tained at 28 °C on a 14/10 hour light/dark cycle. Adults were crossed and eggs promptly collected in Optical Sectioning EM3 media4. -

Whole-Genome Microarray Detects Deletions and Loss of Heterozygosity of Chromosome 3 Occurring Exclusively in Metastasizing Uveal Melanoma
Anatomy and Pathology Whole-Genome Microarray Detects Deletions and Loss of Heterozygosity of Chromosome 3 Occurring Exclusively in Metastasizing Uveal Melanoma Sarah L. Lake,1 Sarah E. Coupland,1 Azzam F. G. Taktak,2 and Bertil E. Damato3 PURPOSE. To detect deletions and loss of heterozygosity of disease is fatal in 92% of patients within 2 years of diagnosis. chromosome 3 in a rare subset of fatal, disomy 3 uveal mela- Clinical and histopathologic risk factors for UM metastasis noma (UM), undetectable by fluorescence in situ hybridization include large basal tumor diameter (LBD), ciliary body involve- (FISH). ment, epithelioid cytomorphology, extracellular matrix peri- ϩ ETHODS odic acid-Schiff-positive (PAS ) loops, and high mitotic M . Multiplex ligation-dependent probe amplification 3,4 5 (MLPA) with the P027 UM assay was performed on formalin- count. Prescher et al. showed that a nonrandom genetic fixed, paraffin-embedded (FFPE) whole tumor sections from 19 change, monosomy 3, correlates strongly with metastatic death, and the correlation has since been confirmed by several disomy 3 metastasizing UMs. Whole-genome microarray analy- 3,6–10 ses using a single-nucleotide polymorphism microarray (aSNP) groups. Consequently, fluorescence in situ hybridization were performed on frozen tissue samples from four fatal dis- (FISH) detection of chromosome 3 using a centromeric probe omy 3 metastasizing UMs and three disomy 3 tumors with Ͼ5 became routine practice for UM prognostication; however, 5% years’ metastasis-free survival. to 20% of disomy 3 UM patients unexpectedly develop metas- tases.11 Attempts have therefore been made to identify the RESULTS. Two metastasizing UMs that had been classified as minimal region(s) of deletion on chromosome 3.12–15 Despite disomy 3 by FISH analysis of a small tumor sample were found these studies, little progress has been made in defining the key on MLPA analysis to show monosomy 3. -
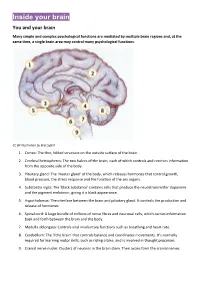
Inside Your Brain You and Your Brain
Inside your brain You and your brain Many simple and complex psychological functions are mediated by multiple brain regions and, at the same time, a single brain area may control many psychological functions. CC BY Illustration by Bret Syfert 1. Cortex: The thin, folded structure on the outside surface of the brain. 2. Cerebral hemispheres: The two halves of the brain, each of which controls and receives information from the opposite side of the body. 3. Pituitary gland: The ‘master gland’ of the body, which releases hormones that control growth, blood pressure, the stress response and the function of the sex organs. 4. Substantia nigra: The ‘black substance’ contains cells that produce the neurotransmitter dopamine and the pigment melatonin, giving it a black appearance. 5. Hypothalamus: The interface between the brain and pituitary gland. It controls the production and release of hormones. 6. Spinal cord: A large bundle of millions of nerve fibres and neuronal cells, which carries information back and forth between the brain and the body. 7. Medulla oblongata: Controls vital involuntary functions such as breathing and heart rate. 8. Cerebellum: The ‘little brain’ that controls balance and coordinates movements. It’s normally required for learning motor skills, such as riding a bike, and is involved in thought processes. 9. Cranial nerve nuclei: Clusters of neurons in the brain stem. Their axons form the cranial nerves. Your brain underpins who you are. It stores your knowledge and memories, gives you the capacity for thought and emotion, and enables you to control your body. The brain is just one part of the nervous system. -

The Strain Rates in the Brain, Brainstem, Dura, and Skull Under Dynamic Loadings
Mathematical and Computational Applications Article The Strain Rates in the Brain, Brainstem, Dura, and Skull under Dynamic Loadings Mohammad Hosseini-Farid 1,2,* , MaryamSadat Amiri-Tehrani-Zadeh 3, Mohammadreza Ramzanpour 1, Mariusz Ziejewski 1 and Ghodrat Karami 1 1 Department of Mechanical Engineering, North Dakota State University, Fargo, ND 58104, USA; [email protected] (M.R.); [email protected] (M.Z.); [email protected] (G.K.) 2 Department of Orthopedic Surgery, Mayo Clinic, Rochester, MN 55905, USA 3 Department of Computer Science, North Dakota State University, Fargo, ND 58104, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-7012315859 Received: 7 March 2020; Accepted: 5 April 2020; Published: 7 April 2020 Abstract: Knowing the precise material properties of intracranial head organs is crucial for studying the biomechanics of head injury. It has been shown that these biological tissues are significantly rate-dependent; hence, their material properties should be determined with respect to the range of deformation rate they experience. In this paper, a validated finite element human head model is used to investigate the biomechanics of the head in impact and blast, leading to traumatic brain injuries (TBI). We simulate the head under various directions and velocities of impacts, as well as helmeted and unhelmeted head under blast shock waves. It is demonstrated that the strain rates for the brain 1 are in the range of 36 to 241 s− , approximately 1.9 and 0.86 times the resulting head acceleration under impacts and blast scenarios, respectively. The skull was found to experience a rate in the range 1 of 14 to 182 s− , approximately 0.7 and 0.43 times the head acceleration corresponding to impact and blast cases. -

Induction of Motor Neurons by Sonic Hedgehog Is Independent of Floor Plate Differentiation Yasuto Tanabe, Henk Roelink and Thomas M
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Induction of motor neurons by Sonic hedgehog is independent of floor plate differentiation Yasuto Tanabe, Henk Roelink and Thomas M. Jessell Howard Hughes Medical Institute, Deptartment of Biochemistry and Molecular Biophysics, Center for Neurobiology and Behavior, Columbia University, 701 West 168th Street, New York, New York 10032, USA. Background: The differentiation of floor plate cells and transfected with Shh induced both floor plate cells and motor neurons in the vertebrate neural tube appears to be motor neurons when grown in contact with neural plate induced by signals from the notochord. The secreted pro- explants, whereas only motor neurons were induced when tein encoded by the Sonic hedgehog (Shh) gene is expressed the explants were grown at a distance from Shh-trans- by axial midline cells and can induce floor plate cells in fected COS cells. Direct transfection of neural plate cells vivo and in vitro. Motor neurons can also be induced in with an Shh-expression construct induced both floor plate vitro by cells that synthesize Sonic hedgehog protein cells and motor neurons, with motor neuron differentia- (Shh). It remains unclear, however, if the motor-neuron- tion occurring prior to, or coincidentally with, floor plate inducing activity of Shh depends on the synthesis of a dis- differentiation. The induction of motor neurons appears, tinct signaling molecule by floor plate cells. To resolve therefore, not to depend on floor plate differentiation. this issue, we have developed an in vitro assay which Conclusions: The induction of motor neurons by uncouples the notochord-mediated induction of motor Shh does not depend on distinct floor-plate-derived neurons from floor plate differentiation, and have used signaling molecules. -

Speed and Segmentation Control Mechanisms Characterized in Rhythmically- Active Circuits Created from Spinal Neurons Produced Fr
RESEARCH ARTICLE Speed and segmentation control mechanisms characterized in rhythmically- active circuits created from spinal neurons produced from genetically-tagged embryonic stem cells Matthew J Sternfeld1,2, Christopher A Hinckley1, Niall J Moore1, Matthew T Pankratz1, Kathryn L Hilde1,3, Shawn P Driscoll1, Marito Hayashi1,2, Neal D Amin1,3,4, Dario Bonanomi1†, Wesley D Gifford1,4,5, Kamal Sharma6, Martyn Goulding7, Samuel L Pfaff1* 1Gene Expression Laboratory, Howard Hughes Medical Institute, Salk Institute for Biological Studies, La Jolla, United States; 2Biological Sciences Graduate Program, University of California, San Diego, La Jolla, United States; 3Biomedical Sciences Graduate Program, University of California, San Diego, La Jolla, United States; 4Medical Scientist Training Program, University of California, San Diego, La Jolla, United States; 5Neurosciences Graduate Program, University of California, San Diego, La Jolla, United States; 6Department of Anatomy and Cell Biology, University of Illinois at Chicago, Chicago, United States; 7Molecular Neurobiology Laboratory, Salk Institute for Biological Studies, La Jolla, United States *For correspondence: pfaff@salk. edu Abstract Flexible neural networks, such as the interconnected spinal neurons that control Present address: †Division of distinct motor actions, can switch their activity to produce different behaviors. Both excitatory (E) Neuroscience, San Raffaele and inhibitory (I) spinal neurons are necessary for motor behavior, but the influence of recruiting Scientific Institute, Milan, Italy different ratios of E-to-I cells remains unclear. We constructed synthetic microphysical neural networks, called circuitoids, using precise combinations of spinal neuron subtypes derived from Competing interests: The mouse stem cells. Circuitoids of purified excitatory interneurons were sufficient to generate authors declare that no oscillatory bursts with properties similar to in vivo central pattern generators. -

Oligodendrocytes in Development, Myelin Generation and Beyond
cells Review Oligodendrocytes in Development, Myelin Generation and Beyond Sarah Kuhn y, Laura Gritti y, Daniel Crooks and Yvonne Dombrowski * Wellcome-Wolfson Institute for Experimental Medicine, Queen’s University Belfast, Belfast BT9 7BL, UK; [email protected] (S.K.); [email protected] (L.G.); [email protected] (D.C.) * Correspondence: [email protected]; Tel.: +0044-28-9097-6127 These authors contributed equally. y Received: 15 October 2019; Accepted: 7 November 2019; Published: 12 November 2019 Abstract: Oligodendrocytes are the myelinating cells of the central nervous system (CNS) that are generated from oligodendrocyte progenitor cells (OPC). OPC are distributed throughout the CNS and represent a pool of migratory and proliferative adult progenitor cells that can differentiate into oligodendrocytes. The central function of oligodendrocytes is to generate myelin, which is an extended membrane from the cell that wraps tightly around axons. Due to this energy consuming process and the associated high metabolic turnover oligodendrocytes are vulnerable to cytotoxic and excitotoxic factors. Oligodendrocyte pathology is therefore evident in a range of disorders including multiple sclerosis, schizophrenia and Alzheimer’s disease. Deceased oligodendrocytes can be replenished from the adult OPC pool and lost myelin can be regenerated during remyelination, which can prevent axonal degeneration and can restore function. Cell population studies have recently identified novel immunomodulatory functions of oligodendrocytes, the implications of which, e.g., for diseases with primary oligodendrocyte pathology, are not yet clear. Here, we review the journey of oligodendrocytes from the embryonic stage to their role in homeostasis and their fate in disease. We will also discuss the most common models used to study oligodendrocytes and describe newly discovered functions of oligodendrocytes. -

Pterosaurs Flight in the Age of Dinosaurs Now Open 2 News at the Museum 3
Member Magazine Spring 2014 Vol. 39 No. 2 Pterosaurs Flight in the Age of Dinosaurs now open 2 News at the Museum 3 From the After an unseasonably cold, snowy winter, will work to identify items from your collection, More than 540,000 Marine Fossils the Museum is pleased to offer a number of while also displaying intriguing specimens from President springtime opportunities to awaken the inner the Museum’s own world-renowned collections. Added to Paleontology Collection naturalist in us all. This is the time of year when Of course, fieldwork and collecting have Ellen V. Futter Museum scientists prepare for the summer been hallmarks of the Museum’s work since Collections at a Glance field season as they continue to pursue new the institution’s founding. What has changed, discoveries in their fields. It’s also when Museum however, is technology. With a nod to the many Over nearly 150 years of acquisitions and Members and visitors can learn about their ways that technology is amplifying how scientific fieldwork, the Museum has amassed preeminent own discoveries during the annual Identification investigations are done, this year, ID Day visitors collections that form an irreplaceable record Day in Theodore Roosevelt Memorial Hall. can learn how scientists use digital fabrication of life on Earth. Today, 21st-century tools— Held this year on May 10, Identification Day to aid their research and have a chance to sophisticated imaging techniques, genomic invites visitors to bring their own backyard finds have their own objects scanned and printed on analyses, programs to analyze ever-growing and curios for identification by Museum scientists. -

Deconstructing Spinal Interneurons, One Cell Type at a Time Mariano Ignacio Gabitto
Deconstructing spinal interneurons, one cell type at a time Mariano Ignacio Gabitto Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy under the Executive Committee of the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2016 © 2016 Mariano Ignacio Gabitto All rights reserved ABSTRACT Deconstructing spinal interneurons, one cell type at a time Mariano Ignacio Gabitto Abstract Documenting the extent of cellular diversity is a critical step in defining the functional organization of the nervous system. In this context, we sought to develop statistical methods capable of revealing underlying cellular diversity given incomplete data sampling - a common problem in biological systems, where complete descriptions of cellular characteristics are rarely available. We devised a sparse Bayesian framework that infers cell type diversity from partial or incomplete transcription factor expression data. This framework appropriately handles estimation uncertainty, can incorporate multiple cellular characteristics, and can be used to optimize experimental design. We applied this framework to characterize a cardinal inhibitory population in the spinal cord. Animals generate movement by engaging spinal circuits that direct precise sequences of muscle contraction, but the identity and organizational logic of local interneurons that lie at the core of these circuits remain unresolved. By using our Sparse Bayesian approach, we showed that V1 interneurons, a major inhibitory population that controls motor output, fractionate into diverse subsets on the basis of the expression of nineteen transcription factors. Transcriptionally defined subsets exhibit highly structured spatial distributions with mediolateral and dorsoventral positional biases. These distinctions in settling position are largely predictive of patterns of input from sensory and motor neurons, arguing that settling position is a determinant of inhibitory microcircuit organization.