Relative Atomic Weights and the Periodic Table
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Isotopes Ions Electronic Structure Relative Atomic Mass (Ar) Chemical
Chemistry 1: Atomic structure and the periodic table Electronic Structure Atoms are ny, too small to see. They have a radius of 0.1 nanometres ( 1 x 10 –10 m) Atoms 1st shell– Lowest energy level and can hold 2 electrons Atoms have no charge because they have the same number of protons and electrons. 2nd shell– Energy level can hold up to 8 electrons Electron Proton 3rd shell onwards– Can hold up to 8 electrons. Nucleus Neutron Electron structure and the periodic table Elements in the same group have the same number of electrons on their outer shell. Electron Orbit around nucleus in Mass Number : shells protons + neutrons Proton number = Electron Number Proton Found in the nucleus Atomic number: Number of neutrons= Neutron Found in the nucleus Protons Mass number—Atomic number An ion is an atom that has lost or gained electrons. An isotope is an atom that has the same number Ions Isotopes of protons but a different number of neutrons. In an ion the number of protons is not equal to the number of electrons so the atom has an overall They have the same atomic number but different atomic mass numbers. charge. This can either be posive or negave. Relative atomic mass (Ar) Relave atomic = sum of (isotope abundance x isotope mass number) Mass (Ar) sum of abundance of all the isotopes An average mass of an element that has a number of different isotopes. Chemical Equations Balancing equaons: There must always be the same number of atoms on Chemical reacons are shown using: both sides of a symbol equaon. -

C H E M I S T
Post-16 Induction C GCSE to A Level Chemistry - Bridging work - H Name : _______________________________ E M I ALL sections should be completed Bring this booklet to your first chemistry lesson in September. S T R Y 1 | P a g e INTRODUCTION Welcome to chemistry. In choosing to study chemistry you will develop many useful lifelong transferable skills, in addition to developing a deeper understanding of the subject. This booklet is designed to prepare you for your first year of study and hopefully answer some of the questions you may have at this stage. The first section gives some useful information about the subject, the details of your course, and gives you the opportunity to see course overview. You will typically have two chemistry teachers; (covering organic and inorganic modules), and lessons will take place in the Lycett building (GL0.02 to GL0.05). Contact me (subject leader) by e-mailing [email protected] over the summer if you have any questions. The second section (from page 6) is designed to review some of the work you will have covered at GCSE and make links with the new material you will cover in your first year of A-level chemistry. A lot of the material we cover in year 12 chemistry you will recognise from GCSE, however, we will build on, and extend your knowledge and understanding in each area. It would be advantageous for you to complete this booklet over the summer (use the answers at the back to self-mark) so that your subject knowledge is fresh when you start in the autumn. -

Atomic Weights and Isotopic Abundances*
Pure&App/. Chem., Vol. 64, No. 10, pp. 1535-1543, 1992. Printed in Great Britain. @ 1992 IUPAC INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY INORGANIC CHEMISTRY DIVISION COMMISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANCES* 'ATOMIC WEIGHT' -THE NAME, ITS HISTORY, DEFINITION, AND UNITS Prepared for publication by P. DE BIEVRE' and H. S. PEISER2 'Central Bureau for Nuclear Measurements (CBNM), Commission of the European Communities-JRC, B-2440 Geel, Belgium 2638 Blossom Drive, Rockville, MD 20850, USA *Membership of the Commission for the period 1989-1991 was as follows: J. R. De Laeter (Australia, Chairman); K. G. Heumann (FRG, Secretary); R. C. Barber (Canada, Associate); J. CCsario (France, Titular); T. B. Coplen (USA, Titular); H. J. Dietze (FRG, Associate); J. W. Gramlich (USA, Associate); H. S. Hertz (USA, Associate); H. R. Krouse (Canada, Titular); A. Lamberty (Belgium, Associate); T. J. Murphy (USA, Associate); K. J. R. Rosman (Australia, Titular); M. P. Seyfried (FRG, Associate); M. Shima (Japan, Titular); K. Wade (UK, Associate); P. De Bi&vre(Belgium, National Representative); N. N. Greenwood (UK, National Representative); H. S. Peiser (USA, National Representative); N. K. Rao (India, National Representative). Republication of this report is permitted without the need for formal IUPAC permission on condition that an acknowledgement, with full reference together with IUPAC copyright symbol (01992 IUPAC), is printed. Publication of a translation into another language is subject to the additional condition of prior approval from the relevant IUPAC National Adhering Organization. ’Atomic weight‘: The name, its history, definition, and units Abstract-The widely used term “atomic weight” and its acceptance within the international system for measurements has been the subject of debate. -

Nuclear Criticality Safety Engineer Training Module 1 1
Nuclear Criticality Safety Engineer Training Module 1 1 Introductory Nuclear Criticality Physics LESSON OBJECTIVES 1) to introduce some background concepts to engineers and scientists who do not have an educational background in nuclear engineering, including the basic ideas of moles, atom densities, cross sections and nuclear energy release; 2) to discuss the concepts and mechanics of nuclear fission and the definitions of fissile and fissionable nuclides. NUCLEAR CRITICALITY SAFETY The American National Standard for Nuclear Criticality Safety in Operations with Fissionable Materials Outside Reactors, ANSI/ANS-8.1 includes the following definition: Nuclear Criticality Safety: Protection against the consequences of an inadvertent nuclear chain reaction, preferably by prevention of the reaction. Note the words: nuclear - related to the atomic nucleus; criticality - can it be controlled, will it run by itself; safety - protection of life and property. DEFINITIONS AND NUMBERS What is energy? Energy is the ability to do work. What is nuclear energy? Energy produced by a nuclear reaction. What is work? Work is force times distance. 1 Developed for the U. S. Department of Energy Nuclear Criticality Safety Program by T. G. Williamson, Ph.D., Westinghouse Safety Management Solutions, Inc., in conjunction with the DOE Criticality Safety Support Group. NCSET Module 1 Introductory Nuclear Criticality Physics 1 of 18 Push a car (force) along a road (distance) and the car has energy of motion, or kinetic energy. Climb (force) a flight of steps (distance) and you have energy of position relative to the first step, or potential energy. Jump down the stairs or out of a window and the potential energy is changed to kinetic energy as you fall. -

Mendeleev's Periodic Table of the Elements the Periodic Table Is Thus
An Introduction to General / Inorganic Chemistry Mendeleev’s Periodic Table of the Elements Dmitri Mendeleev born 1834 in the Soviet Union. In 1869 he organised the 63 known elements into a periodic table based on atomic masses. He predicted the existence and properties of unknown elements and pointed out accepted atomic weights that were in error. The periodic table is thus an arrangement of the elements in order of increasing atomic number. Elements are arranged in rows called periods. Elements with similar properties are placed in the same column. These columns are called groups. An Introduction to General / Inorganic Chemistry The modern day periodic table can be further divided into blocks. http://www.chemsoc.org/viselements/pages/periodic_table.html The s, p, d and f blocks This course only deals with the s and p blocks. The s block is concerned only with the filling of s orbitals and contains groups I and II which have recently been named 1 and 2. The p block is concerned only with the filling of p orbitals and contains groups III to VIII which have recently been named 13 to 18. An Introduction to General / Inorganic Chemistry Groups exist because the electronic configurations of the elements within each group are the same. Group Valence Electronic configuration 1 s1 2 s2 13 s2p1 14 s2p2 15 s2p3 16 s2p4 17 s2p5 18 s2p6 The type of chemistry exhibited by an element is reliant on the number of valence electrons, thus the chemistry displayed by elements within a given group is similar. Physical properties Elemental physical properties can also be related to electronic configuration as illustrated in the following four examples: An Introduction to General / Inorganic Chemistry 1. -

Answer Key Chapter 6: Standard Review Worksheet 1
Answer Key Chapter 6: Standard Review Worksheet 1. 1 amu = 1.66 _ 10–24 g. For example, the average atomic mass of sodium is 22.99 amu, which represents the average mass of all the sodium atoms in the world (including all the various isotopes and their relative abundances). So that we will be able to use the mass of a sample of sodium to count the number of atoms of sodium present in the sample, we consider that every sodium atom in a sample has exactly the same mass (the average atomic mass). The average atomic mass of an element is typically not a whole number of amu’s because of the presence of the different isotopes of the element, each with its own relative abundance. Since the relative abundance of an element can be any number, when the weighted average atomic mass of the element is calculated, the average is unlikely to be a whole number. 2. On a microscopic basis, one mole of a substance represents Avogadro’s number (6.022 _ 1023) of individual units (atoms or molecules) of the substance. On a macroscopic basis, one mole of a substance represents the amount of substance present when the molar mass of the substance in grams is taken (for example, 12.01 g of carbon will be one mole of carbon). 3. The molar mass of a compound is the mass in grams of one mole of the compound (6.022 _ 1023 molecules of the compound) and is calculated by summing the average atomic masses of all the atoms present in a molecule of the compound. -

5.1: Atomic Mass Unit 5.1: Atomic Mass
Topic 5: Stoichiometry - Chemical Arithmetic Masses of some atoms: 1 -24 16 -23 1H = 1.6736×10 g 8 O = 2.6788 ×10 g 238 -22 92U = 3.9851×10 g Introducing…….the Atomic Mass Unit (amu) 1 amu = 1.66054 x 10-24 g 1 5.1: Atomic Mass Unit Atomic Mass is defined relative to Carbon -12 isotope 12 12 amu is the mass of the 6 C isotope of carbon Carbon -12 atom = 12.000 amu Hydrogen -1 atom = 1.008 amu Oxygen -16 atom = 15.995 amu Chlorine -35 atom = 34.969 amu 2 5.1: Atomic Mass - Natural Abundance We deal with the naturally occurring mix of isotopes, rather than pure isotopes Carbon has three natural isotopes Isotope Mass (amu) Abundance (%) 12C 12.000 98.892 13C 13.00335 1.108 14C 14.00317 1 x 10-4 Any shovelful of Carbon from living material will have a Naturally Occurring Abundance of 98.892% 12C, 1.108% 13C and 0.0001% 14C 3 1 5.1: Atomic Mass - Relative Abundance How do we take into account the naturally occurring Abundances? Take the average mass of the various isotopes weighted according to their Relative Abundances Relative Isotope Mass (amu) Abundance (%) Abundance 12C 12.000 98.892 0.98892 13C 13.00335 1.108 0.0108 -6 14C 14.00317 1 x 10-4 1 x 10 N.B. The % Abundance adds up to 100 The Relative Abundance adds up to 1 4 5.1: Average Atomic Mass Relative Isotope Mass (amu) Abundance (%) Abundance 12C 12.000 98.892 0.98892 13C 13.00335 1.108 0.0108 -6 14C 14.00317 1 x 10-4 1 x 10 The Average Atomic Mass is given by: (0.98892 x 12.000 amu) + (0.01108 x 13.00335 amu) + (1 x 10-6 x 14.00317 amu) = 12.011 amu 5 5.1: Atomic and Molecular Mass You can calculate the mass of any compound from the sum of the atomic masses from the periodic table. -

Atomic Structure the Periodic Table Amount of Substance Bonding And
1 | C h e m i s t r y Contents 2(a) Atomic Structure 3 2(b) The Periodic Table 14 2(c) Amount of Substance 18 2(d) Bonding and Structure 28 2(e) Enthalpy Changes 39 2 | C h e m i s t r y 2(a) – Atomic Structure Scientists working in any area of chemical industry or research require a firm understanding of atomic structure and electron configurations and their use in providing the fundamental basis for chemical structures and reactions. Radiographers, environmental chemists and archaeologists all make use of specific isotopes in their work. Analytical chemists use UV/visible spectra and flame emission spectra to help characterise substances and colorimetry as a quantitative analytical technique. The origin of colour in compounds is of great importance in the dye-, pigment-, and paint-based industries and to development chemists researching new products. The Atom All elements are made of atoms. Atoms are made up of 3 types of particle. These are protons, neutrons and electrons. Electrons have -1 charge. They move around the nucleus in orbitals, which take up most of the volume of the atom. Most of the mass of the atom is concentrated in the nucleus. The nucleus is very small in comparison to the whole atom. The nucleus is where you find the protons and neutrons. The mass and charge of these subatomic particles is really small, so relative mass and relative charge are used instead. The table shows the relative masses and charges of protons, neutrons and electrons. Subatomic particle Relative mass Relative Charge Proton 1 +1 Neutron 1 0 Electron 1/2000 -1 3 | C h e m i s t r y Atomic Number and Mass Number You can figure out the number of protons, neutrons and electrons in an atom from the nuclear symbol. -

Make an Atom Vocabulary Grade Levels
MAKE AN ATOM Fundamental to physical science is a basic understanding of the atom. Atoms are comprised of protons, neutrons, and electrons. Protons and neutrons are at the center of the atom while electrons live in lobe-shaped clouds outside the nucleus. The number of electrons usually matches the number of protons, yielding a net neutral charge for the atom. Sometimes an atom has less neutrons or more neutrons than protons. This is called an isotope. If an atom has different numbers of electrons than protons, then it is an ion. If an atom has different numbers of protons, it is a different element all together. Scientists at Idaho National Laboratory study, create, and use radioactive isotopes like Uranium 234. The 234 means this isotope has an atomic mass of 234 Atomic Mass Units (AMU). GRADE LEVELS: 3-8 VOCABULARY Atom – The basic unit of a chemical element. Proton – A stable subatomic particle occurring in all atomic nuclei, with a positive electric charge equal in magnitude to that of an electron, but of opposite sign. Neutron – A subatomic particle of about the same mass as a proton but without an electric charge, present in all atomic nuclei except those of ordinary hydrogen. Electron – A stable subatomic particle with a charge of negative electricity, found in all atoms and acting as the primary carrier of electricity in solids. Orbital – Each of the actual or potential patterns of electron density that may be formed on an atom or molecule by one or more electrons. Ion – An atom or molecule with a net electric charge due to the loss or gain of one or more electrons. -

Using Your Periodic Tables (Inside Back Cover of Revision Guide)
Group Staff email 1st point of contact – [email protected] 9Q1 [email protected] 9Q2 [email protected] 9Q3 [email protected] 9Q4 [email protected] Week 1 Q band Rev. links Task guide pages F Task 1 Lesson 1 – Electronic structure Click Q1. Write down as much as you can tell me about A, B and C. 96 105 Periodic Q2. What element are they? Identify each one. table Q3. What equation do we use to calculate the relative atomic mass 97 of an element? Using your periodic tables (inside back cover of revision Periodic guide), complete the following… table Q1. What is the symbol for Lithium? (inside Q2. Which element has the symbol Na? back Q3. What is the atomic number of Carbon? cover) Q4. What is the mass number of Oxygen? Q5. What element has an atomic number of 4? Q6. Which element has a mass number of 14? Q7. Name 3 elements in group 1. Q8. Name 3 elements in Group 7. Q9. Give the symbol for Neon. Q10. What is the proton number for Chlorine? Copy and complete: Key Word Definition A_____ The smallest part of an element that can exist. Periodic Helps you s____ p_________ in table p____________ G_______ Vertical columns in the periodic table. P_______ Found in the nucleus of an atom. It has a positive charge. Electron N________ charge and very little mass. Periodic table Draw the electron arrangement for the first 20 elements (inside using pencil. Some of these as examples are found on the back pages given → cover) Use periodic table to help you and relevant pages → 105 105 Task 2 Lesson 2 – the periodic table Click Q1. -

Molar Mass 1 Molar Mass
Molar mass 1 Molar mass In chemistry, the molar mass is a physical property. It is defined as the mass of a given substance (chemical element or chemical compound) divided by its amount of substance. The base SI unit for molar mass is kg/mol. However, for historical reasons, molar masses are almost always expressed in g/mol. As an example, the molar mass of water is approximately: M(H O) ≈ 18 g⋅mol−1 2 Molar masses of elements The molar mass of atoms of an element is given by the atomic mass of the element multiplied by the molar mass constant, M −3 u = 1×10 kg/mol = 1 g/mol: M(H) = 1.007 97(7) × 1 g/mol = 1.007 97(7) g/mol M(S) = 32.065(5) × 1 g/mol = 32.065(5) g/mol M(Cl) = 35.453(2) × 1 g/mol = 35.453(2) g/mol M(Fe) = 55.845(2) × 1 g/mol = 55.845(2) g/mol. Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: atomic weights are dimensionless quantities (i.e., pure numbers) whereas molar masses have units (in this case, grams/mole). Some elements are usually encountered as molecules, e.g. hydrogen (H 2), sulfur (S 8), chlorine (Cl 2). The molar mass of molecules of these elements is the molar mass of the atoms multiplied by the number of atoms in each molecule: M(H 2) = 2 × 1.007 97(7) × 1 g/mol = 2.015 88(14) g/mol M(S 8) = 8 × 32.065(5) × 1 g/mol = 256.52(4) g/mol M(Cl 2) = 2 × 35.453(2) × 1 g/mol = 70.906(4) g/mol. -
How Atoms Differ A
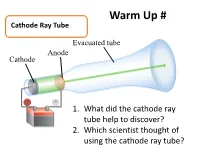
Warm Up # Cathode Ray Tube Evacuated tube Anode Cathode − + 1. What did the cathode ray tube help to discover? Battery 2. Which scientist thought of using the cathode ray tube? How Atoms Differ a. Properties of Subatomic Particles Particle Symbol Location Relative Relative Actual Charge mass mass (g) outside 1 Electron e- the 9.11 x nucleus -1 1840 10-28 g in the 1.673 x Proton p+ nucleus +1 1 10-24 g in the 1.675 x Neutron 0 n nucleus 0 1 10-24 g Elements on the Periodic Table b. Atomic Number • the number of protons in an atom • Identifies element c. Mass Number •represents the total number of protons and neutrons in the nucleus Mass number A ZX atomic number d. Isotopes • Atoms that have the same number of protons but have a different masses • Ex: 3 isotopes of carbon: 12 13 14 6C 6C 6C e. Average Atomic Mass • the weighted average of the isotopes of that element. • Formula: % mass % Atomic mass abundance of abundance mass of ( ) ( of ) = of x Isotope + of x + … an element Isotope #2 Isotope #1 #1 Isotope #2 Average Atomic Mass • The mass of an atom is so small it is difficult to work with, so chemists have developed an atomic standard to compare all the masses • The standard is the atomic mass unit (amu) • If the mass of an element is not close to a whole number, it is because the atom has several isotopes • The atomic mass is the weighted average of the isotopes of that element Example 1 Silver has two naturally occurring isotopes.