Chiton Myogenesis 105
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
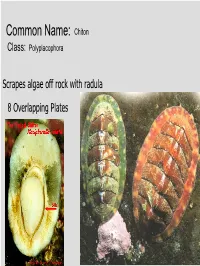
Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

The Chiton Radula: a Unique Model for Biomineralization Studies
4 The Chiton Radula: A Unique Model for Biomineralization Studies Lesley R. Brooker1 and Jeremy A. Shaw2 1University of the Sunshine Coast 2Centre for Microscopy, Characterisation & Analysis University of Western Australia Australia 1. Introduction Over the course of evolution, a range of strategies have been developed by different organisms to produce unique materials and structures perfected for their specific function. This biological mastery of materials production has inspired the birth of the new discipline of biomaterials through biomimicry (Birchall, 1989). Chitons (Mollusca: Polyplacophora) are slow moving, bilaterally symmetrical and dorso- ventrally flattened molluscs that are commonly found on hard substrata in intertidal regions of coastlines around the world (Kaas & Jones, 1998). All species are characterized by a series of eight dorsal, articulating shell plates or valves, which may be embedded, to varying degrees, in a fleshy, muscular girdle (Kaas & Jones, 1998) (Figure 1). Approximately 750 living species are known, and while intertidal regions are home to the majority of chitons, a number of species can be found at depths of up to 8000m where they feed on detrital material (Kaas & Jones, 1998). Fig. 1. Photograph of the dorsal surface of the chiton Acanthopleura gaimardi, showing the eight overlapping aragonite plates surrounded by the fleshy girdle, which, in this species, is covered in small aragonite spines. Chitons feed by rasping macro- and micro-algae from the rocks on which they live through the use of a radula. The radula has been coined as a conveyor belt of continuously developing www.intechopen.com 66 Advanced Topics in Biomineralization teeth, replaced by new teeth as they are worn and lost. -

Marine Invertebrate Field Guide
Marine Invertebrate Field Guide Contents ANEMONES ....................................................................................................................................................................................... 2 AGGREGATING ANEMONE (ANTHOPLEURA ELEGANTISSIMA) ............................................................................................................................... 2 BROODING ANEMONE (EPIACTIS PROLIFERA) ................................................................................................................................................... 2 CHRISTMAS ANEMONE (URTICINA CRASSICORNIS) ............................................................................................................................................ 3 PLUMOSE ANEMONE (METRIDIUM SENILE) ..................................................................................................................................................... 3 BARNACLES ....................................................................................................................................................................................... 4 ACORN BARNACLE (BALANUS GLANDULA) ....................................................................................................................................................... 4 HAYSTACK BARNACLE (SEMIBALANUS CARIOSUS) .............................................................................................................................................. 4 CHITONS ........................................................................................................................................................................................... -

Phylum MOLLUSCA Chitons, Bivalves, Sea Snails, Sea Slugs, Octopus, Squid, Tusk Shell
Phylum MOLLUSCA Chitons, bivalves, sea snails, sea slugs, octopus, squid, tusk shell Bruce Marshall, Steve O’Shea with additional input for squid from Neil Bagley, Peter McMillan, Reyn Naylor, Darren Stevens, Di Tracey Phylum Aplacophora In New Zealand, these are worm-like molluscs found in sandy mud. There is no shell. The tiny MOLLUSCA solenogasters have bristle-like spicules over Chitons, bivalves, sea snails, sea almost the whole body, a groove on the underside of the body, and no gills. The more worm-like slugs, octopus, squid, tusk shells caudofoveates have a groove and fewer spicules but have gills. There are 10 species, 8 undescribed. The mollusca is the second most speciose animal Bivalvia phylum in the sea after Arthropoda. The phylum Clams, mussels, oysters, scallops, etc. The shell is name is taken from the Latin (molluscus, soft), in two halves (valves) connected by a ligament and referring to the soft bodies of these creatures, but hinge and anterior and posterior adductor muscles. most species have some kind of protective shell Gills are well-developed and there is no radula. and hence are called shellfish. Some, like sea There are 680 species, 231 undescribed. slugs, have no shell at all. Most molluscs also have a strap-like ribbon of minute teeth — the Scaphopoda radula — inside the mouth, but this characteristic Tusk shells. The body and head are reduced but Molluscan feature is lacking in clams (bivalves) and there is a foot that is used for burrowing in soft some deep-sea finned octopuses. A significant part sediments. The shell is open at both ends, with of the body is muscular, like the adductor muscles the narrow tip just above the sediment surface for and foot of clams and scallops, the head-foot of respiration. -

Polyplacophora: Chitonidae): First Records in European Waters
Zootaxa 3626 (4): 593–596 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Correspondence ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3626.4.14 http://zoobank.org/urn:lsid:zoobank.org:pub:00EE2336-D60C-49A1-BC40-0FAE551F5DB6 Tonicia atrata and Chiton cumingsii (Polyplacophora: Chitonidae): First records in European waters ANDRÉS ARIAS1,2 & NURIA ANADÓN1 1Departamento de Biología de Organismos y Sistemas (Zoología), Universidad de Oviedo, Oviedo 33071, Spain 2Corresponding author. E-mail: [email protected] At present, over 300 species of marine alien Mollusca are reported from the European waters (Streftaris et al. 2005; Zenetos et al. 2010). However, only three alien polyplacophoran have been recorded: Chaetopleura angulata (Spengler, 1797), Acanthopleura gemmata (Blainville, 1825) and Chiton hululensis (E. A. Smith, 1903); the latter is considered as “questionable” (Zenetos et al. 2010). These polyplacophoran constituting about 1% of the alien marine mollusc reported from Europe. Here we present the first record of Tonicia atrata (Sowerby, 1840) and Chiton cumingsii Frembly, 1827 in European waters, constituting the first evidence of their presence outside their native range. Furthermore, we give brief notes on the taxonomy and distribution of T. atrata and C. cumingsii, and discuss the potential pathways for introduction to Europe. In Europe, T. atrata occurs together with the well-known alien Ch. angulata; and probably both species have historically been misidentified in collections because both reach large size (> 60 mm) and in many cases the larger size was commonly used to differentiate the presumed alien (Ch. angulata) from the native polyplacophoran of smaller size. -

Limpets and Chitons, Teacher Guide
TEACHER BACKGROUND Unit 5 - Limpets and Chitons Limpets and Chitons (Ki tons) Key Concepts 1. Limpets are single shelled marine animals that use a flat, muscular foot to remain attached to rocks. 2. Chitons are marine animals which have eight shell plates for protection and use a flat, muscular foot to remain attached to rocks. 3. Both limpets and chitons use their rasping tongue or radula to graze on tiny algae which covers the rocks in tidepools. Background Limpets and chitons are common and commonly overlooked tidepool animals. We tend to think of free swimming fish and scurrying crabs when we think of marine animals. However, a great many marine animals, such as limpets and chitons, lead a sedentary existence, firmly affixed to the bottom or some other feature. Both limpets and chitons possess a large, muscular foot which they use for attachment. During low tides, most limpets and chitons stay in one spot. When submerged during high tides, they glide slowly over the rocks. As the tide falls, they return to precisely the same spot. For limpets that spot is a neat “scar” the exact size and shape of the limpet. Limpets and chitons eat very small but nevertheless visible (macroscopic) plants (algae). Using a rasping tongue, called a radula, a limpet or chiton grazes the surface scraping off the microscopic plants as it travels. This feeding action underscores the interrelationships that exist between plants and animals. The existence of any species of animal is directly or indirectly related to the existence of some species of plant. Although both molluscs, the shells of limpets and chitons are quite different. -

A Molecular Phylogeny of the Patellogastropoda (Mollusca: Gastropoda)
^03 Marine Biology (2000) 137: 183-194 ® Spnnger-Verlag 2000 M. G. Harasevvych A. G. McArthur A molecular phylogeny of the Patellogastropoda (Mollusca: Gastropoda) Received: 5 February 1999 /Accepted: 16 May 2000 Abstract Phylogenetic analyses of partiaJ J8S rDNA formia" than between the Patellogastropoda and sequences from species representing all living families of Orthogastropoda. Partial 18S sequences support the the order Patellogastropoda, most other major gastro- inclusion of the family Neolepetopsidae within the su- pod groups (Cocculiniformia, Neritopsma, Vetigastro- perfamily Acmaeoidea, and refute its previously hy- poda, Caenogastropoda, Heterobranchia, but not pothesized position as sister group to the remaining Neomphalina), and two additional classes of the phylum living Patellogastropoda. This region of the Í8S rDNA Mollusca (Cephalopoda, Polyplacophora) confirm that gene diverges at widely differing rates, spanning an order Patellogastropoda comprises a robust clade with high of magnitude among patellogastropod lineages, and statistical support. The sequences are characterized by therefore does not provide meaningful resolution of the the presence of several insertions and deletions that are relationships among higher taxa of patellogastropods. unique to, and ubiquitous among, patellogastropods. Data from one or more genes that evolve more uni- However, this portion of the 18S gene is insufficiently formly and more rapidly than the ISSrDNA gene informative to provide robust support for the mono- (possibly one or more -

GUMBOOT CHITON Cryptochiton Stelleri Middendorff, 1846 (Acanthochitonidae)
GUMBOOT CHITON Cryptochiton stelleri Middendorff, 1846 (Acanthochitonidae) Global rank G5 (26Jun2006) State rank S5 (26Jun2006) State rank reasons Overall population and trends unknown, but the species is considered locally abundant and widespread in coastal areas. Threatened by human harvest; low recruitment rates make the species vulnerable to overharvest. There is also concern about contamination as a result of individuals are rarely observed (MacGinitie and coastal development and oil spills and the MacGinitie 1968). potential effects of climatic warming. Ecology TaxonomyRecent work by Okusu et al. (2003) Very few predators; they include the lurid places the genus Cryptochiton in a subclade rocksnail (Ocinebrina lurida), tidepool sculpin within the Acanthochitonina along with Tonicella, (Oligocottus maculosus), river otter (Lontra Mopalia, and Katharina, based on genetic and canadensis; O’Clair and O’Clair 1998) and the morphological similarities. large asteroid (Pycnopodia helianthoides; Yates 1989). A traditional source of food for humans, General description but the meat is very tough (Harbo 1997, O’Clair The largest chiton in the world, up to 33 cm long. and O’Clair 1998). The purple urchin In Southeast Alaska, typically smaller, about 15 (Strongylocentrotus purpuratus) and red urchin cm (Yates 1989, O’Clair and O’Clair 1998). (S. franciscanus) may compete with the gumboot Species is unique among chitons because all chiton for space and food (Yates 1989). May be eight plates are completely concealed by the an indirect commensal to coralline algae by thick and leathery reddish brown or brown mantle eating the fleshy red algae that grows on its (Field and Field 1999, Cowles 2005). The surface and reducing the negative effects of underside is yellow or orange, with a broad foot algae overgrowth (Yates 1989). -

An Annotated Checklist of the Marine Macroinvertebrates of Alaska David T
NOAA Professional Paper NMFS 19 An annotated checklist of the marine macroinvertebrates of Alaska David T. Drumm • Katherine P. Maslenikov Robert Van Syoc • James W. Orr • Robert R. Lauth Duane E. Stevenson • Theodore W. Pietsch November 2016 U.S. Department of Commerce NOAA Professional Penny Pritzker Secretary of Commerce National Oceanic Papers NMFS and Atmospheric Administration Kathryn D. Sullivan Scientific Editor* Administrator Richard Langton National Marine National Marine Fisheries Service Fisheries Service Northeast Fisheries Science Center Maine Field Station Eileen Sobeck 17 Godfrey Drive, Suite 1 Assistant Administrator Orono, Maine 04473 for Fisheries Associate Editor Kathryn Dennis National Marine Fisheries Service Office of Science and Technology Economics and Social Analysis Division 1845 Wasp Blvd., Bldg. 178 Honolulu, Hawaii 96818 Managing Editor Shelley Arenas National Marine Fisheries Service Scientific Publications Office 7600 Sand Point Way NE Seattle, Washington 98115 Editorial Committee Ann C. Matarese National Marine Fisheries Service James W. Orr National Marine Fisheries Service The NOAA Professional Paper NMFS (ISSN 1931-4590) series is pub- lished by the Scientific Publications Of- *Bruce Mundy (PIFSC) was Scientific Editor during the fice, National Marine Fisheries Service, scientific editing and preparation of this report. NOAA, 7600 Sand Point Way NE, Seattle, WA 98115. The Secretary of Commerce has The NOAA Professional Paper NMFS series carries peer-reviewed, lengthy original determined that the publication of research reports, taxonomic keys, species synopses, flora and fauna studies, and data- this series is necessary in the transac- intensive reports on investigations in fishery science, engineering, and economics. tion of the public business required by law of this Department. -

The External Morphology of the Gill of Patella Caerulea L
D. AKŞİT, B. FALAKALI MUTAF Turk J Zool 2011; 35(4): 603-606 © TÜBİTAK Short Communication doi:10.3906/zoo-0907-82 Th e external morphology of the gill of Patella caerulea L. (Mollusca: Gastropoda) Deniz AKŞİT*, Beria FALAKALI MUTAF Akdeniz University, Faculty of Aquatic Sciences and Fisheries, Dumlupınar Bulvarı, 07059 Antalya - TURKEY Received: 17.07.2009 Abstract: Gross morphology of the gill was studied using a scanning electron microscope (SEM) in order to associate it with its function in genus Patella. Secondary gills consisted of a single layer of gill folds, which appear mostly as triangular leafl ets in scanning electron micrographs. Th e lamellae showed a prominent thickening on the free margin and horizontal swellings, which bear a series of ciliary structures on the frontal surface, as well as disperse bunches. Th ey diff ered from the discocilia and, because chemoreception has been attributed to them in the absence of an osphradium, they are thought to contribute to the primary respiratory role of the gills. Th ese morphological characteristics are to be researched further, both in natural and experimental specimens of this genus. Key words: Patella, gill, SEM, ciliary structures Patella caerulea L. (Mollusca: Gastropoda) solungaçlarının dış morfolojisi Özet: Patella genusunun genel solungaç morfolojisi, fonksiyonuyla ilişkilendirilerek taramalı elekron mikroskobunda incelenmiştir. İkincil solungaç katlantıları taramalı elektron mikroskobunda genellikle üçgenimsi yapraklar şeklinde görülen tek bir tabakadan oluşmaktadır. Herbir yaprağın serbest kenarlarında belirgin bir kalınlaşma ve ön yüzeyinde yatay kabartılarda bir dizi dağınık siller halinde bulunmaktadır. Bu demetler diskosiliadan farklıdır ve kimyasal algılayıcı osfradyumun olmaması nedeniyle, solungaçın birincil görevi solunuma katkıda bulunduğu düşünülmektedir. -

Patellid Limpets: an Overview of the Biology and Conservation of Keystone Species of the Rocky Shores
Chapter 4 Patellid Limpets: An Overview of the Biology and Conservation of Keystone Species of the Rocky Shores Paulo Henriques, João Delgado and Ricardo Sousa Additional information is available at the end of the chapter http://dx.doi.org/10.5772/67862 Abstract This work reviews a broad spectrum of subjects associated to Patellid limpets’ biology such as growth, reproduction, and recruitment, also the consequences of commercial exploitation on the stocks and the effects of marine protected areas (MPAs) in the biology and populational dynamics of these intertidal grazers. Knowledge of limpets’ biological traits plays an important role in providing proper background for their effective man- agement. This chapter focuses on determining the effect of biotic and abiotic factors that influence these biological characteristics and associated geographical patterns. Human exploitation of limpets is one of the main causes of disturbance in the intertidal ecosys- tem and has occurred since prehistorical times resulting in direct and indirect alterations in the abundance and size structure of the target populations. The implementation of MPAs has been shown to result in greater biomass, abundance, and size of limpets and to counter other negative anthropogenic effects. However, inefficient planning and lack of surveillance hinder the accomplishment of the conservation purpose of MPAs. Inclusive conservation approaches involving all the stakeholders could guarantee future success of conservation strategies and sustainable exploitation. This review also aims to estab- lish how beneficial MPAs are in enhancing recruitment and yield of adjacent exploited populations. Keywords: Patellidae, limpets, fisheries, MPAs, conservation 1. Introduction The Patellidae are one of the most successful families of gastropods that inhabit the rocky shores from the supratidal to the subtidal, a marine habitat subject to some of the most © 2017 The Author(s). -

Patellogastropod Molluscs Support Multiple Invasions of Deep Sea Habitats
240 Patellogastropod Molluscs Support Multiple Invasions of Deep Sea Habitats LINDBERG, David R.; GURALNICK*, Robert; HEDEGf\ARD, Claus, Dept. of Integrative Biology & Museum ofPaleontolobJ]', University ofCalifomia, Berkeley, CA 94720, U.S.A.; JACOBS, David, Dept. ofBiology, University ofCalifomia, Los Angeles, CA 90095, U.S.A. The Patellogastropoda are the most primitive of all Gastropoda based on both morphological and molecular data. Moreover, they are almost exclusively an intertidal and shallow water group. Over 90% ofthe species are found in 33 m or less. Patellogastropod characters are well known and have been sufficiently studied so that autapomorphies are unlikely to deeply masked relationships. Moreover, shell structure, preserved in both fossil and Recent taxa, is character rich and ecologically invariant. Patellogastropods are the sister taxon of all other gastropods and are therefore assumed to be an ancient lineage. Four patellogastropod taxa have representatives in the deep sea.. Patellogastropod taxa at vents and seeps have been previously argued to have ridden vents down from shallow water habitats or are evidence of immigration and colonization from shallow water habitats (onshore - offshore model). Vent fauna could also be aggregates ofsome 'old' shallow water things that rode the vents down, and colonization from both deep sea and shallow water habitats. It is difficult to test different alternative hypothesis. Arguments for antiquity of patellogastropods in the deep sea are often based on fossil occurrences of taxonomically similar taxa. This similarity not rigorously tested to determine whether or not it reflects relatedness. Most taxonomies, especially for fossil and living marine invertebrates, do not, but instead have been based on comparison of shell morphologies between fossil and living gastropods.