LC-MS/MS Screening of Phenolic Compounds in Wild and Cultivated Grapes Vitis Amurensis Rupr
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
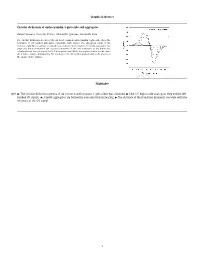
Graphical Abstract Circular Dichroism of Anthocyanidin 3-Glucoside Self
Graphical abstract Circular dichroism of anthocyanidin 3-glucoside self-aggregates Raquel Gavara, Vesselin Petrov, Alexandre Quintas, Fernando Pina * The circular dichroism spectra of the six most common anthocyanidin 3-glucoside show the formation of left handed aggregates compatible with dimers. The absorption bands of the monomer split by increasing concentration according to the formation of H and J aggregates. The angle and distance between the transition moments of the two monomers in the dimer was calculated from the splitting of the 0–0 absorption band. While the angle is similar for the series the distance changes dramatically. The intensity of the CD signal is proportional to the inverse of the square of the distance. Highlights Q10 " The circular dichroism spectra of six common anthocyanins 3-glucosides was obtained. " Like 3,5-diglucoside analogous they exhibit left- handed CD signals. " J and H aggregates are formed by concentration increasing. " The distance of the transition moments correlate with the intensity of the CD signal. 1 1 2 Circular dichroism of anthocyanidin 3-glucoside self-aggregates a a b a,⇑ 3 Q1 Raquel Gavara , Vesselin Petrov , Alexandre Quintas , Fernando Pina 4 a REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829516 Monte de Caparica, Portugal 5 b Instituto Superior de Ciencias da Saude Egas Moniz, Centro de Investigação Interdisciplinar Egas Moniz, P-2829511 Monte de Caparica, Caparica, Portugal 6 7 article info a b s t r a c t 1 9 10 Self-association constants for the flavylium cations of the six most common anthocyanidin 3-glucosides 20 11 were determined by circular dichroism (CD) and UV–Vis spectroscopy. -

Electrochemical and Antioxidant Properties of Anthocyanins and Anthocyanidins
CROATICA CHEMICA ACTA CCACAA 80 (1) 29¿34 (2007) ISSN-0011-1643 CCA-3135 Original Scientific Paper Electrochemical and Antioxidant Properties of Anthocyanins and Anthocyanidins Andréia A. de Lima, Eliana M. Sussuchi, and Wagner F. De Giovani* Departamento de Química, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Av. Bandeirantes 3900, 14040-901, Ribeirão Preto – SP, Brazil RECEIVED JUNE 26, 2006; REVISED SEPTEMBER 29, 2006; ACCEPTED OCTOBER 27, 2006 Electrochemical properties of delphinidin, cyanidin, pelargonidin, kuromanin and callistephin were investigated by cyclic and differential pulse voltammetries at different pH values and also in methanol. On the basis of oxidation potentials, the order of antioxidant activity for antho- cyanidins is delphinidin > cyanidin > pelargonidin. Oxidation peaks for anthocyanins (kuro- manin and callistephin) are shifted to more positive potentials compared to anthocyanidins (delphinidin, cyanidin and pelargonidin). Oxidation peak currents are linearly dependent on the Keywords square root of the scan rate, which is typical of a diffusion controlled electrochemical process. anthocyanins Antioxidant activities of the compounds were evaluated using the 1,1-diphenyl-2-picrylhydra- anthocyanidins zyl (DPPH) radical-scavenging method and they are directly related to their redox potential antioxidants values. The order of the antioxidant activity is delphinidin > cyanidin > pelargonidin > kuro- electrochemical properties manin > callistephin. INTRODUCTION pharmacological properties that render them interesting potential cancer chemopreventive agents.9–11 Because Anthocyanins are one of the main classes of flavonoids anthocyanins are widely consumed, finding out addi- in red wines. They have been pointed out as the molecu- tional biological activities related to these compounds lar entities that are most likely responsible for what has would be of great interest. -

Callistephin Enhances the Protective Effects of Isoflurane on Microglial Injury Through Downregulation of Inflammation and Apoptosis
802 MOLECULAR MEDICINE REPORTS 20: 802-812, 2019 Callistephin enhances the protective effects of isoflurane on microglial injury through downregulation of inflammation and apoptosis LILI ZHAO, SHIBIAO CHEN, TIANYIN LIU, XIUHONG WANG, HAIJIN HUANG and WEICHENG LIU Department of Anesthesiology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, P.R. China Received June 18, 2018; Accepted March 15, 2019 DOI: 10.3892/mmr.2019.10282 Abstract. Microglia are the major immune cells in the central enhanced the effects of isoflurane. Callistephin may therefore nervous system. Microglial activation can be beneficial or constitute a candidate drug agent that may target inflammatory detrimental depending on the stimuli and the physiopathological and growth regulatory signaling pathways, thus ameliorating environment. Microglial activation is involved in a variety certain aspects of neurodegenerative diseases. of neurodegenerative disorders. Different anesthetic agents have exhibited diverse effects on microglial activation and Introduction the engulfment process. The anthocyanin callistephin has been demonstrated to have antioxidant and anti‑inflammatory Microglial cells are the major immune cell in the central properties, and these were assessed in the present study, with a nervous system (CNS), responding against types of endog- focus on its effect on microglial activation. Mouse microglial enous and exogenous stimuli, including infection by bacteria, cells C8-4B were treated with 100 ng/µl lipopolysaccharide viruses, prions and β-amyloid plaques (1). Microglia are (LPS) and 1 ng/µl interferon-γ. Cells were subsequently treated activated upon exposure to different stimuli and, depending with 2% isoflurane, 100 µM callistephin or both. LPS promoted on the environmental context, this may be beneficial or detri- apoptosis in C8-B4 cells, and this was reduced following mental to the functionality and physiology of the CNS (2). -

AVALUACIÓ DE COMPOSTOS FENÒLICS EN ALIMENTS MITJANÇANT TÈCNIQUES HPLC-DAD I UHPLC-DAD-Msn
AVALUACIÓ DE COMPOSTOS FENÒLICS EN ALIMENTS MITJANÇANT TÈCNIQUES HPLC-DAD I UHPLC-DAD-MSn Albert RIBAS AGUSTÍ Dipòsit legal: Gi. 955-2013 http://hdl.handle.net/10803/116771 ADVERTIMENT. L'accés als continguts d'aquesta tesi doctoral i la seva utilització ha de respectar els drets de la persona autora. Pot ser utilitzada per a consulta o estudi personal, així com en activitats o materials d'investigació i docència en els termes establerts a l'art. 32 del Text Refós de la Llei de Propietat Intel·lectual (RDL 1/1996). Per altres utilitzacions es requereix l'autorització prèvia i expressa de la persona autora. En qualsevol cas, en la utilització dels seus continguts caldrà indicar de forma clara el nom i cognoms de la persona autora i el títol de la tesi doctoral. No s'autoritza la seva reproducció o altres formes d'explotació efectuades amb finalitats de lucre ni la seva comunicació pública des d'un lloc aliè al servei TDX. Tampoc s'autoritza la presentació del seu contingut en una finestra o marc aliè a TDX (framing). Aquesta reserva de drets afecta tant als continguts de la tesi com als seus resums i índexs. ADVERTENCIA. El acceso a los contenidos de esta tesis doctoral y su utilización debe respetar los derechos de la persona autora. Puede ser utilizada para consulta o estudio personal, así como en actividades o materiales de investigación y docencia en los términos establecidos en el art. 32 del Texto Refundido de la Ley de Propiedad Intelectual (RDL 1/1996). Para otros usos se requiere la autorización previa y expresa de la persona autora. -

Characterisation of Bioactive Compounds in Berries from Plants Grown Under Innovative Photovoltaic Greenhouses
Journal of Berry Research 8 (2018) 55–69 55 DOI:10.3233/JBR-170258 IOS Press Characterisation of bioactive compounds in berries from plants grown under innovative photovoltaic greenhouses Federica Blandoa,∗, Carmela Gerardia, Massimiliano Rennab,c, Sergio Castellanod and Francesco Seriob aInstitute of Sciences of Food Production (ISPA), CNR, Lecce, Italy bInstitute of Sciences of Food Production (ISPA), CNR, Bari, Italy cDepartment of Agricultural and Environmental Science, University of Bari Aldo Moro, Bari, Italy dDepartment of Science of Agriculture, Food and Environment, (SAFE) University of Foggia, Foggia, Italy Received 2 July 2017; accepted 1 November 2017 Abstract. BACKGROUND: Bioactive compounds, mainly polyphenols, present in berries, are thought to be responsible for the health benefits of these fruit. Therefore, it is worthwhile to define the optimal environmental conditions to maximise their polyphenol content. OBJECTIVE: With the aim to define the optimal conditions for berry cultivation in an innovative environment, red rasp- berry, wild strawberry and blackberry plants were grown in a traditional greenhouse in comparison with two photovoltaic greenhouses with different shading area. METHODS: Hydroalcoholic extracts of ripe berries were evaluated by HPLC analysis, for their anthocyanins, organic acids and sugar contents. Moreover, phenolic content (by the Folin-Ciocalteu assay) and antioxidant activity (by the Trolox equivalent antioxidant capacity-TEAC assay) were assayed on the same berry extracts. RESULTS: Total anthocyanins, -

First-Pass Metabolism of Polyphenols from Selected Berries: a High-Throughput Bioanalytical Approach
antioxidants Article First-Pass Metabolism of Polyphenols from Selected Berries: A High-Throughput Bioanalytical Approach Francisco J. Olivas-Aguirre 1,*, Sandra Mendoza 2, Emilio Alvarez-Parrilla 3 , Gustavo A. Gonzalez-Aguilar 4 , Monica A. Villegas-Ochoa 4 , Jael T.J. Quintero-Vargas 1 and Abraham Wall-Medrano 3,* 1 Departamento de Ciencias de la Salud, Universidad de Sonora (Campus Cajeme), Blvd Bordo Nuevo s/n, Ejido Providencia, Cd, Obregón 85199, Mexico; [email protected] 2 Departamento de Investigación y Posgrado en Alimentos (PROPAC), Facultad de Química, Universidad Autónoma de Querétaro, Cerro de las Campanas s/n, Santiago de Querétaro 76010, Mexico; [email protected] 3 Instituto de Ciencias Biomédicas, Universidad Autónoma de Ciudad Juárez, Anillo Envolvente del PRONAF y Estocolmo s/n, Ciudad Juárez 32310, Mexico; [email protected] 4 Centro de Investigación en Alimentación y Desarrollo, A.C. (CIAD), Carretera a Ejido La Victoria, Km. 0.6, Hermosillo 83304, Mexico; [email protected] (G.A.G.-A.); [email protected] (M.A.V.-O.) * Correspondence: [email protected] (F.J.O.-A.); [email protected] (A.W.-M.) Received: 29 March 2020; Accepted: 11 April 2020; Published: 13 April 2020 Abstract: Small berries are rich in polyphenols whose first-pass metabolism may alter their ultimate physiological effects. The antioxidant capacity and polyphenol profile of three freeze-dried berries (blackberry, raspberry, Red Globe grape) were measured and their apparent permeability (Papp) and first-pass biotransformation were tracked with an ex vivo bioanalytical system [everted gut sac (rat) + three detection methods: spectrophotometry, HPLC-ESI-QTOF-MS, differential pulse voltammetry (DPV)]. -

Antioxidant Molecules from Plant Waste: Extraction Techniques and Biological Properties
Antioxidant Molecules from Plant Waste: Extraction Techniques and Biological Properties Authors: Cynthia E. Lizárraga-Velázquez, Nayely Leyva-López, Crisantema Hernández, Erick Paul Gutiérrez-Grijalva, Jesús A. Salazar-Leyva, Idalia Osuna-Ruíz, Emmanuel Martínez-Montaño, Javier Arrizon, Abraham Guerrero, Asahel Benitez-Hernández, Anaguiven Ávalos-Soriano Date Submitted: 2021-06-21 Keywords: residues, green technologies, fruit, vegetable, valorization, Extraction, bioactive peptides, terpenes, phenolic compounds, phytosterols Abstract: The fruit, vegetable, legume, and cereal industries generate many wastes, representing an environmental pollution problem. However, these wastes are a rich source of antioxidant molecules such as terpenes, phenolic compounds, phytosterols, and bioactive peptides with potential applications mainly in the food and pharmaceutical industries, and they exhibit multiple biological properties including antidiabetic, anti-obesity, antihypertensive, anticancer, and antibacterial properties. The aforementioned has increased studies on the recovery of antioxidant compounds using green technologies to value plant waste, since they represent more efficient and sustainable processes. In this review, the main antioxidant molecules from plants are briefly described and the advantages and disadvantages of the use of conventional and green extraction technologies used for the recovery and optimization of the yield of antioxidant naturals are detailed; finally, recent studies on biological properties of antioxidant molecules -

Production of Native Plants for Seed, Biomass, and Natural Products A
Production of native plants for seed, biomass, and natural products A Dissertation SUBMITTED TO THE FACULTY OF UNIVERSITY OF MINNESOTA BY Katrina Franziska Freund Saxhaug IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Craig C. Sheaffer, advisor March 2020 © Katrina Franziska Freund Saxhaug 2019 Acknowledgements The research presented in this document would not have been possible without the love and support of countless mentors, colleague, friends and family. Foremost, I am forever grateful to my advisor, Dr. Craig Sheaffer, whose direction, support, understanding, and unending generosity made it possible for me to complete my doctorate. I am also eternally thankful for my unofficial co-advisor, Dr. Adrian Hegeman, for his kindness, intellectual brilliance, and support throughout my degree program. I am also incredibly grateful to Dr. Susan Galatowitsch and Dr. Clay Carter for their guidance, wisdom, and constructive and insightful commentaries. Though not on my committee, Dr. Jacob Jungers was incredibly generous with his time, advice, and support in all aspects of this research. While a doctoral student, I was supported by grants from the Minnesota Department of Agriculture through the AGRI Crop Research Grant Program and the Specialty Crop Block Grant Program. Additional support came through the Minnesota Institute for Sustainable Agriculture gift fund, graciously provided by Leanna Forcier. Further support was provided by the University of Minnesota, including the Hueg-Harrison Graduate Fellowship, the Mark and Jean Schroepfer Fellowship, the Nancy Jo Ehlke Fellowship, and Annie’s Sustainable Agriculture Scholarship. The Sustainable Cropping Systems Lab, under the direction of Dr Craig Sheaffer, and the Plant Metabolomics Lab, under the direction of Dr. -

Characterization of Phenolic Compounds in Highly-Consumed Vegetable Matrices by Using Advanced Analytical Techniques
UNIVERSITY OF GRANADA FACULTY OF SCIENCES Department of Analytical Chemistry Research Group FQM-297 “Environmental, Biochemical and Foodstuff Analytical Control” Functional Food Research and Development Center (CIDAF) DOCTORAL THESIS CHARACTERIZATION OF PHENOLIC COMPOUNDS IN HIGHLY-CONSUMED VEGETABLE MATRICES BY USING ADVANCED ANALYTICAL TECHNIQUES CARACTERIZACIÓN DE COMPUESTOS FENÓLICOS EN MATRICES VEGETALES MEDIANTE TÉCNICAS ANALTICALAS AVANZADAS Presented by IBRAHIM M. ABU REIDAH Submitted for a Doctoral degree in Chemistry GRANADA, 2013 Editor: Editorial de la Universidad de Granada Autor: Ibrahim M. Abu Reidah D.L.: GR 1899-2013 ISBN: 978-84-9028-591-6 This doctoral thesis has been conducted through financing from the Ministry of Foregin Affairs of Spain & The Spanish Agency Of International Cooperation for Development (MAEC-AECID) scholarship and funds from the Research Group FQM-297 “Environmental, Biochemical and Foodstuff Analytical Control” (Department of Analytical Chemistry, University of Granada) and Functional Food Research and Development Center (CIDAF) from different projects, contracts and grants from the central and autonomic administrations and research plan of the University of Granada. CHARACTERIZATION OF PHENOLIC COMPOUNDS IN HIGHLY-CONSUMED VEGETABLE MATRICES BY USING ADVANCED ANALYTICAL TECHNIQUES By IBRAHIM M. ABU REIDAH Granada, 2013 Signed by Dr. Alberto Fernández-Gutiérrez Full Professor of the Department of Analytical Chemistry Faculty of Sciences, University of Granada Signed by Dr. Antonio Segura Carretero Full Professor of the Department of Analytical Chemistry Faculty of Sciences, University of Granada Signed by Dr. David Arráez-Román Assistant Professor of the Department of Analytical Chemistry Faculty of Sciences, University of Granada Submitted for a Doctoral Degree in Chemistry Signed by Ibrahim M. -

Effect of Different Anthocyanidin Glucosides on Lutein Uptake By
molecules Article Effect of Different Anthocyanidin Glucosides on Lutein Uptake by Caco-2 Cells, and Their Combined Activities on Anti-Oxidation and Anti-Inflammation In Vitro and Ex Vivo Minh Anh Thu Phan 1,*, Martin Bucknall 2 and Jayashree Arcot 1,* ID 1 Food and Health Cluster, School of Chemical Engineering, UNSW, Sydney, NSW 2052, Australia 2 Mark Wainwright Analytical Centre, UNSW, Sydney, NSW 2052, Australia; [email protected] * Correspondence: [email protected] (M.A.T.P.); [email protected] (J.A.); Tel.: +61-2-9385-5360 (J.A.) Academic Editors: Natália Martins and Derek J. McPhee Received: 23 July 2018; Accepted: 11 August 2018; Published: 14 August 2018 Abstract: The interactive effects on anti-oxidation and anti-inflammation of lutein combined with each of the six common anthocyanidin glucosides were studied in both chemical and cellular systems. The combined phytochemicals showed an antagonism in the inhibition of lipid oxidation in a liposomal membrane, but showed an additive effect on cellular antioxidant activity in Caco-2 cells. Lutein was an active lipoxygenase inhibitor at 2–12 µM while anthocyanins were inactive. The concentration of lutein when it was used in combination with anthocyanins was 25–54% higher than when lutein was used alone (i.e., IC50 = 1.2 µM) to induce 50% of lipoxygenase inhibition. Only the combination of lutein with malvidin-3-glucoside showed anti-inflammatory synergy in the suppression of interleukin-8, and the synergy was seen at all three ratios tested. Some mixtures, however, showed anti-inflammatory antagonism. The presence of anthocyanins (5–7.5 µM) did not affect lutein uptake (2.5–5 µM) by Caco-2 cells. -

Changes in Phenolic Compounds and Antioxidant Activity of Fruit Musts and Fruit Wines During Simulated Digestion
molecules Article Changes in Phenolic Compounds and Antioxidant Activity of Fruit Musts and Fruit Wines during Simulated Digestion Tomasz Tarko * , Aleksandra Duda-Chodak and Agata Soszka Department of Fermentation Technology and Microbiology, Faculty of Food Technology, University of Agriculture in Krakow, ul. Balicka 122, 30-149 Krakow, Poland; [email protected] (A.D.-C.); [email protected] (A.S.) * Correspondence: [email protected]; Tel.: +48-12-6624792 Academic Editor: Urszula Gawlik-Dziki Received: 5 November 2020; Accepted: 24 November 2020; Published: 27 November 2020 Abstract: The content of polyphenols (total phenolic content (TPC)) and the antioxidant activity (AOX) of food products depend on the raw materials used and the technological processes in operation, but transformations of these compounds in the digestive tract are very important. The aim of this study was to determine the TPC, profile of polyphenols, and AOX of apple and blackcurrant musts and wines in order to evaluate the changes occurring in a simulated human digestive system. The research material consisted of apples and blackcurrant, from which musts and fruit wines were obtained. All samples were subjected to three-stage digestion in a simulated human digestive system and then analyzed for the following: TPC (Folin–Ciocalteu method) and profile (HPLC), AOX (method with 2,20-azino-bis (3-ethylbenzothiazoline-6-sulfonic) acid (ABTS) radical), and for the wines also total extract, volatile acidity (International Organization of Vine and Wine (OIV) method), and sugar profile (HPLC). The antioxidant activity of fruit wines is directly related to the total polyphenol content. Phenolic compounds were transformed during all digestive stages, which led to the formation of compounds with higher antioxidant capacity. -

Quality Assesment of Urtica Dioica L. Samples Collected from Different Locations of Turkey
Turk J Pharm Sci 11(2), 223-230, 2014 Short communication Quality Assesment of Urtica dioica L. Samples Collected from Different Locations of Turkey Sanem HO§BA§, Mustafa ASLAN*, Ekrem SEZİK Gazi University, Faculty of Pharmacy, Department of Pharmacognosy, 06330 Ankara, TURKEY Urtica dioica L. (Urticacea) is widely distributed throughout the temperate regions of the world. Leaves of the plant recommended not only for the complaints associated with rheumatoid arthritis, cardiovascular diseases and diabetes but also have antiviral, antioxidant, anti-inflammatory activities. It is also used as traditional medicine as panacea in Turkey. Despite, U. dioica leaf is monographed in European Pharmacopeia as “Nettle Leaf - Urtica folium” and standardized by liquid chromatography for its chlorogenic acid content; aerial parts of plants are sold instead of leaves at the herbal shops for medical purposes in Turkey. The objective of our study is to determine quality of samples and compare the chlorogenic acid amount in aerial parts and leaves collected from different localities of Anatolia. Extraction procedure was employed according to European Pharmacopeia 6.0. /?-Coumaric acid was used as internal standart. RP-HPLC was performed in order to determine chlorogenic acid amounts. The leaf samples collected from Düzce, Maraş, Isik Mountain and Palandöken Mountain has possesed highest chlorogenic acid amounts 1.91±0.0003%, 1.156±0.0019%, 0.67±0.00068%, 0.63±0.0024% respectively. However amount of chlorogenic acid in aerial parts of same samples has been found 1.047±0.0007%, 0.751±0.0033%, 0.31±0.0085%, 0.488±0.0051% respectively. Results indicate that in all samples, chlorogenic acid content has been found higher in leaves than aerial parts.